Infections are the most common complication after kidney transplant (KT),1 accounting for between 15 and 20% of mortality in KT.2 Adenoviruses are double-stranded, non-enveloped DNA viruses, whose infection is related to immunosuppression such as in cases of solid organ transplantation.3 Intestine and liver transplants, anti-lymphocyte induction and donor-recipient serological differences are additional risk factors.4 The incidence of asymptomatic viremia in the first year post-KT is 4.1–6.5 % with up to 10% invasive disease.3 The most common effect in KT is hemorrhagic cystitis (HC), whose symptoms mimic a urinary tract infection.3 Its diagnosis entails detecting viral load in samples of the affected organ: the gold standard is histopathology. 5 We present a case of HC secondary to Adenovirus in a patient with simultaneous pancreas-kidney transplant (SPK) and successfully treated with intravesical cidofovir.
Our case study is a 57-year-old woman with type 1 diabetes mellitus and chronic secondary kidney disease, who received a first SPK. We conducted induction therapy with anti-lymphocyte antibodies associated with a maintenance treatment based on tacrolimus, mycophenolic acid (MPA) and corticosteroids. The evolution was favorable, with normal kidney and pancreatic functions.
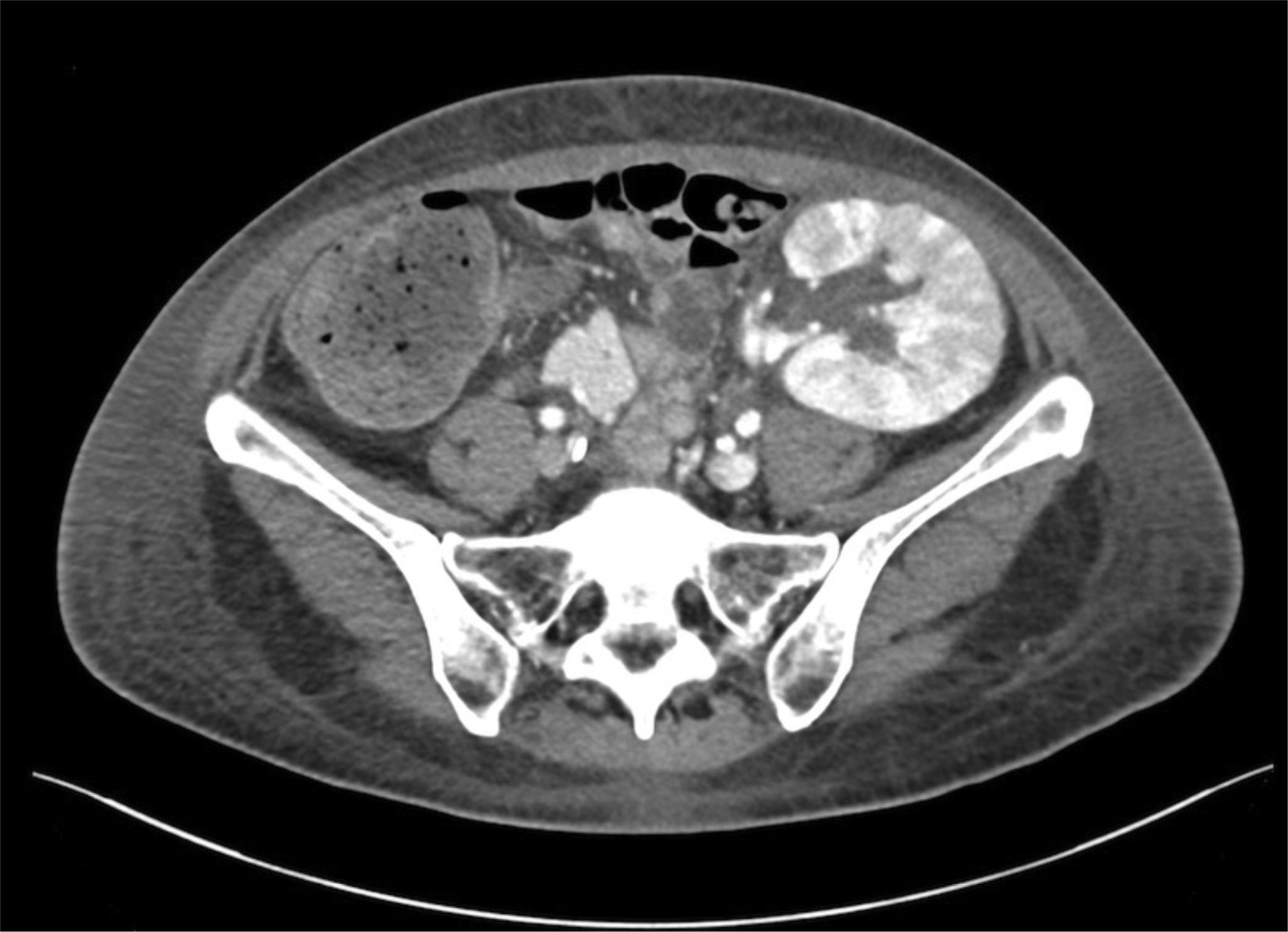
Seven months after the transplant, she was admitted due to a fever with respiratory symptoms lasting one month and macroscopic hematuria. Complementary exams revealed evidence of elevated inflammatory reactants, bicytopenia (anemia and leukopenia) and impaired kidney graft function. Bladder and kidney ultrasound was normal. We first interpreted the case as a respiratory viral infection with added bacterial superinfection, initiating broad spectrum and prophylactic empirical antibiotic treatment with trimethoprim/sulfamethoxazole given the sustained lymphopenia; in addition, we suspended MPA, maintaining the therapy with both immunosuppressants. In the absence of bacteriuria and negativity in the initial microbiological studies (urine culture, blood cultures, sputum culture, PCR of nasopharyngeal frotis for SARS-CoV-2, VRS and Influenzavirus A y B). We expanded the study with a thoracoabdominal computed tomography scan that showed splenomegaly and signs suggestive of nephritis in the renal graft (Fig. 1). The microbiological study was also expanded with respiratory smears and blood PCR for various viruses with positivity for Adenovirus (440,017 copies/mL), as well as its presence in urine (5,071,409 copies/mL), which confirmed HC secondary to Adenovirus. Given the persistence of fever and anemia-leucopenia even after the reduction of immunosuppression, we initiated antiviral therapy with cidofovir.
Cidofovir is an antiviral active against various DNA viruses, including Adenovirus, although its use in KT is limited due to its potential nephrotoxicity. American guidelines recommend its use in severe, resistant or widespread Adenovirus infections,5 but its intravenous administration entails nephrotoxicity risks. However, intravesical administration has shown to be a promising alternative, especially in the treatment of HC due to BK polyomavirus in hematopoietic stem cell transplantation (HSCT), with complete response rates of 88% with no significant adverse effects. In KT, there is a case of this administration route in the literature in a KT recipient with HC and secondary Adenovirus with resolution of clinical signs after intravesical infusion.7 These experiences highlight the potential efficacy of this route of administration in minimizing nephrotoxicity. However, the evidence is still limited, and the optimal dose is not established, varying in the literature between 1 and 5 mg/kg at 60−100 mL of 0.9% NaCl.6 Due the role of cellular immunity as the main actor against viral infections, cell therapy using infusions of specific T lymphocytes versus Adenovirus or multi-virus is under development in hematopoietic stem cell transplantation.8 There is still no data for solid organ transplants.5
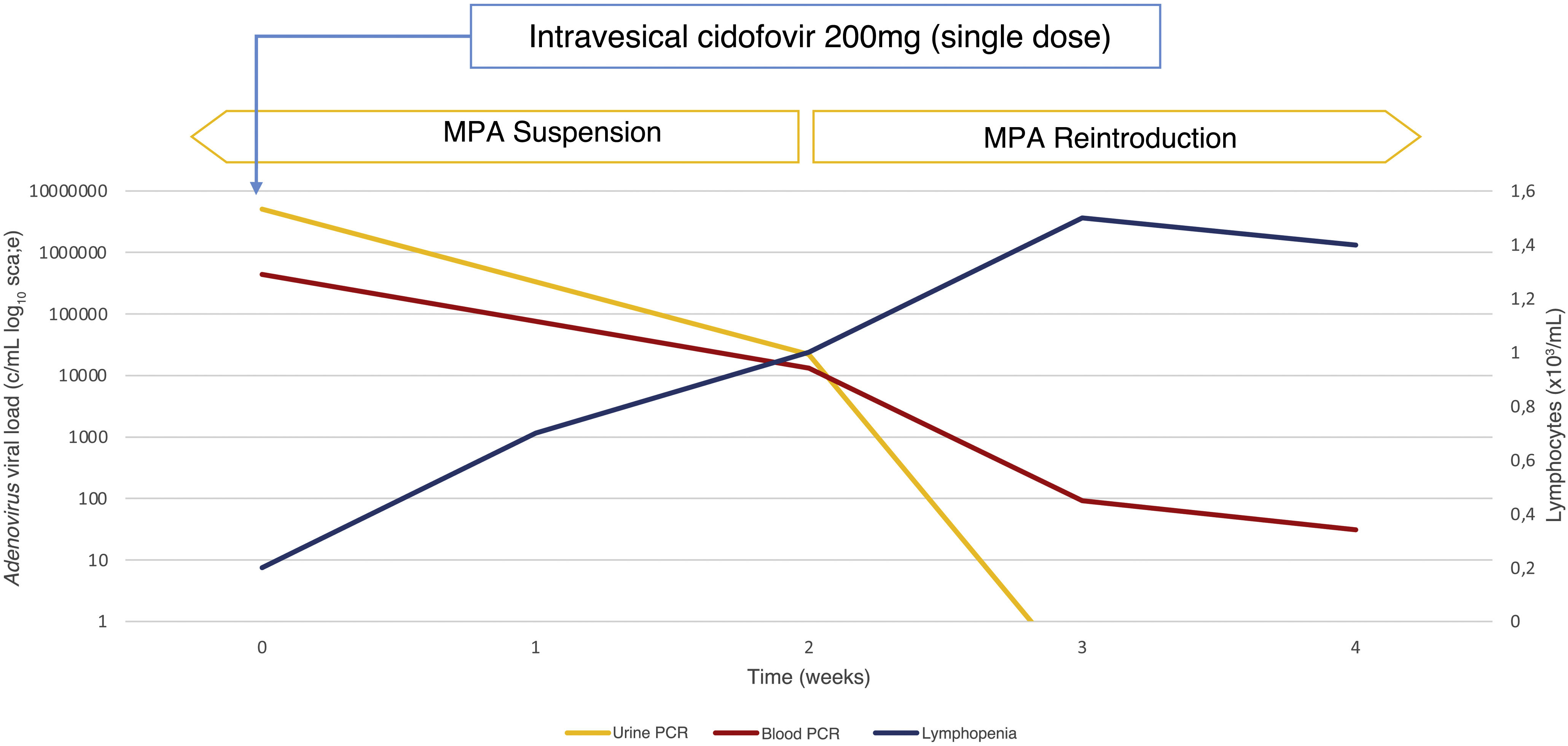
In our case, we chose a single dose of intravesical cidofovir of 200 mg with intravesical duration of 1 h. After treatment, the patient presented clinical improvement, with viremia reduction and reduced viruria of 99.56% one month after treatment (Fig. 2). Subsequently, we restarted MPA at low doses with persistent decline in viruria and maintenance of function renal with improvement of cytopenias and complete resolution of hematuria.
In conclusion, the Adenovirus should be considered in the differential diagnosis of unexplained hematuria in immunocompromised patients, especially if preceded by respiratory symptoms. This case highlights the potential usefulness of intravesical cidofovir in the treatment of HC by Adenovirus in a kidney transplant, providing an effective and less nephrotoxic alternative. Additional studies are required to confirm its safety, efficacy and establish guidelines dosage clearings in this clinical context.
FundingThis research has not received specific support from public sector agencies, commercial sector or non-profit entities.