El trasplante renal (TX) representa el tratamiento de elección de la mayoría de los pacientes con enfermedad renal crónica, pero estos enfermos presentan una elevada mortalidad con respecto a la población general, a pesar de los nuevos tratamientos inmunosupresores y del mejor manejo clínico de estos pacientes. Este hecho justifica que los excelentes resultados obtenidos a corto plazo no lleven una trayectoria paralela a más largo plazo. Esta preocupante situación se debe, probablemente, a una alta prevalencia de entidades cardiovasculares y de procesos infecciosos y tumorales que concurren en esta población en el marco del tratamiento inmunosupresor. Asimismo, existe interacción entre estos procesos, los cuales comparten factores causales y mecanismos patogénicos comunes, incrementando la mortalidad. Por tanto, identificar las causas de muerte y los factores de riesgo, aplicar modelos predictivos de morbilidad y mortalidad e intervenir sobre los factores causales pueden ser algunas de las estrategias para mejorar los resultados de trasplante renal en términos de supervivencia. En esta revisión se analizan algunas de las evidencias que condicionan esta elevada mortalidad tras el TX, así como los aspectos terapéuticos y pronósticos relacionados con la comorbilidad: 1) Magnitud del problema y causas de muerte de estos enfermos; 2) Identificación de los factores de riesgo de mortalidad; 3) Estrategias terapéuticas para disminuir la mortalidad pos-TX; y 4) Predicción de la mortalidad y la enfermedad isquémica cardíaca.
Renal transplantation (TX) is the treatment of choice in the majority of patients with chronic kidney disease. But, these patients have a high mortality rate with respect to the general population despite new immunosuppression treatments and improved clinical management. This justifies that the excellent results obtained in the short terms do not have a parallel clinical benefit in the long term. This worrying situation is probably due to a high prevalence of cardiovascular conditions and infectious and neoplastic entities amongst this population against a backdrop of immunosuppression treatment. Furthermore, there is interaction between these processes, which share causal factors and common pathogenic mechanisms. Mortality thus increases. Therefore, identifying the causes of death and the risk factors, applying morbidity and mortality predictive models and intervening in causal factors could constitute some of the strategies for improving renal transplantation results in terms of survival. This review analyses some of the evidence conditioning this high mortality rate following TX, as well and the therapeutic and prognostic aspects associated with co-morbidity: 1) Magnitude of the problem and causes of death among sufferers; 2) Identification of mortality risk factors; 3) Therapeutic strategies for decrease post-TX mortality and; 4) Prediction of mortality and ischaemic heart disease.
INTRODUCTION
Although kidney transplant (TX) is the treatment of choice for patients with chronic advanced kidney disease, death with a functioning graft constitutes the second most common cause of loss of cadaveric donor grafts. A similar situation can be observed under the best starting conditions, such as live-donor TX.1 This may explain why excellent short-term results do not lead to a parallel path in the medium and long run in terms of survival.2,3 In addition, this mortality with functioning graft has remained stable over the years, despite new therapeutic and optimization strategies in the clinical management of these patients.1,4 Therefore, prolonging the survival of patients following TX is currently a clinical priority. From this, it follows that learning the causes of death, identifying risk factors, implementing predictive models of mortality and comorbidity and intervening on causal factors may be some of the strategies that will optimize TX results. However, it should be asked: Do we know with any accuracy the causal relationship between risk factors and mortality? Are we implementing timely treatments in the correct way so as to prolong survival? Lastly, are we able to predict mortality?
Throughout this review, we will tackle the etiopatogenic evidence associated with this elevated mortality rate and the therapeutic and prognosis aspects of comorbidity inherent in TX, emphasising the following clinical issues: 1) Magnitude of the problem and causes of death among sufferers; 2) Identification of mortality risk factors; 3) Therapeutic strategies for decrease post-TX mortality and; 4) Prediction of mortality and ischaemic heart disease.
1. MAGNITUDE OF THE PROBLEM AND CAUSES OF DEATH
Cohort observational studies have demonstrated that cardiovascular mortality in TX subjects is significantly higher than that of the general population of similar age and sex.5 Indeed, recent data from the Australian register revealed that, while renal TX improves survival rates in comparison with dialysis, these patients have higher global mortality than the general the population.4 To be precise, the global annual mortality rate of these patients hovers between 5% and 7%, increasing to 10% in the population over 65 years of age. This means that premature mortality (during the first year) represents approximately 20% of graft loss, while late mortality - beyond the first year - constitutes 40% of graft loss.
But what are the causes of this mortality? Recent data from the American register confirm that cardiovascular disesase (CVD) is the leading cause of death (30%) among the transplant population, followed very closely by causes of infectious origin (21%).1 Likewise, an observational study of data from the Australian register shows that, although CVD remains the primary cause of death (40%), mortality of infectious origin (34%) has been rising gradually over the years.6 Moreover, a Spanish multicentre observational study of 2600 transplant patients between 2000 and 2002 confirms that the most frequent cause of death is cardiovascular in origin, particularly ischaemic heart disease.7 Lastly, a review of several observational studies produced similar results.8 CVD is the leading cause of mortality in these patients (30%-48%), followed by infectious causes (17%-30%) and neoplasia (8%-18%). That said, there are significant differences between the rates of mortality between different countries. Specifically, in one Latin-American observational study, the risk of death for Spanish patients with TX was significantly higher to that of the Latin Americans, hinting that environmental and ethnic factors may provide the reason for such differences.9
2. IDENTIFICATION OF MORTALITY RISK FACTORS
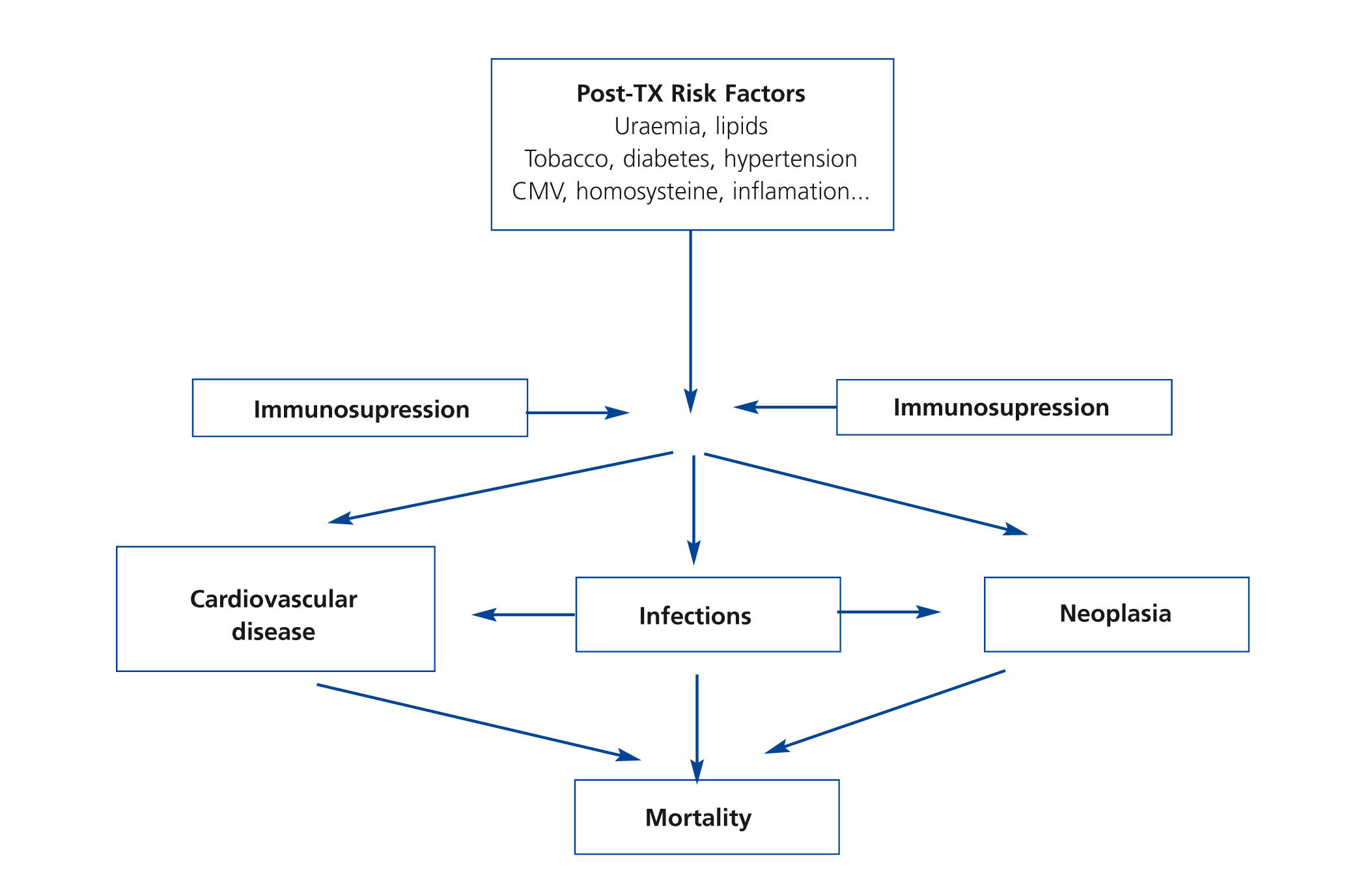
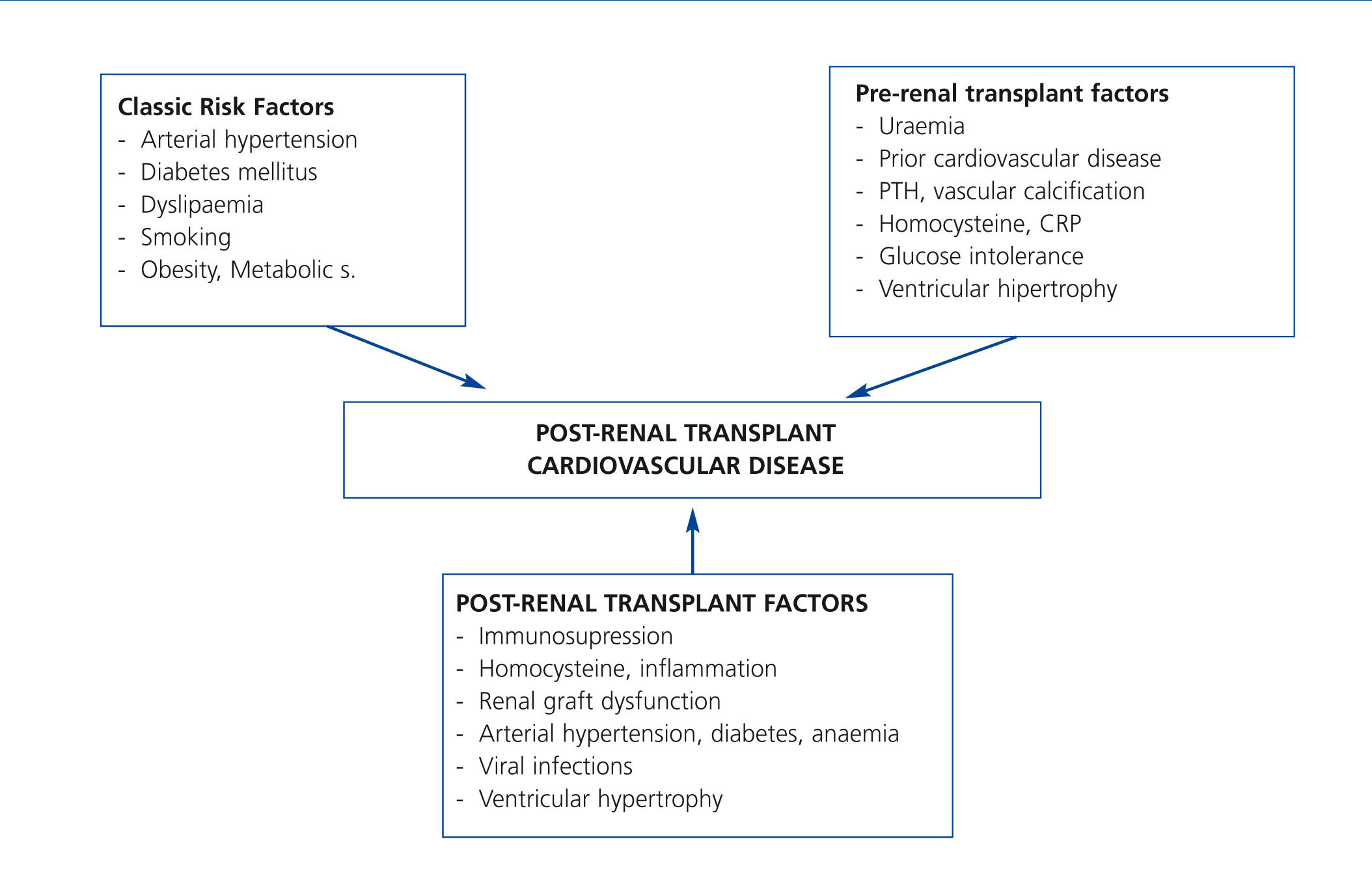
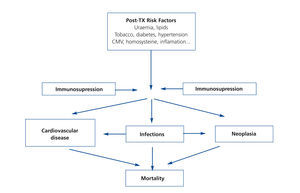
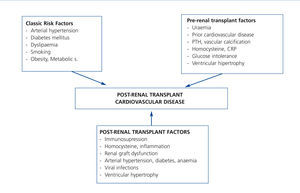
It is very possible that this excessive mortality in the TX population is due to the interaction of great cardiovascular comobidity and infectious and neoplastic processes, which share common causal factors within the framework of immunosuppressive treatment (Figure 1). If we focus on CVD, few clinical entities bring together as many risk factors as TX. Although researchers have identified both pre-TX risk factors and others inherent to the transplant itself (Figure 2); the factors that contribute most to cardiovascular risk are diabetes, arterial hypertension and dyslipidaemia in patients receiving immunosuppressive treatment. Long-term cohort studies of the European population have shown an increase in the incidence of cardiovascular events and greater mortality in relation to both pre-TX and post-TX diabetes, where this entity constitutes an independent risk factor for developing cardiovascular complications.10 It is extremely possible that this is due to a poorer metabolic and vascular profile in patients with post-TX diabetes compared with patients without diabetes reflected in higher serum cholesterol and triglyceride levels, as well as a larger increase in systolic and diastolic blood pressure.11 At the same time, arterial hypertension is highly prevalent in follow-ups (80%) and may have an effect in these patients' lower rate of survival. In fact, hypertension significantly increases the risk of death and graft loss when adjusted for other confounding variables, such as acute rejection or renal function, etc.12. Dyslipidaemia may also contribute to a greater risk of the progression of atheromatosis as well as these patients developing CVD.13 At the same time, immunosuppressive drugs may magnify the deleterious effects of cardiovascular risk factors and so contribute to higher cardiovascular morbidity and mortality rates in this population.8 Taken together, these factors may explain why up to 40% of TX patients suffer some type of cardiovascular event in the first ten years after TX, as it has been possible to show through long-term observational studies in Europe.14
In any case, traditional vascular risk factors do not provide sufficient explanation for elevated cardiovascular mortality. On the one hand, there are risk factors that are shared between CVD and the development of infectious and neoplastic processes, such as immunosuppression, smoking or diabetes, among others. On the other, there is a high prevalence of emergent risk factors like proteinuria, inflammation, renal dysfunction or the presence of vascular calcification that lead to an atypical relationship between TX and CVD. For example, an observational study by Kasiske et al. revealed that the Framingham risk score underestimated the cardiovascular risk in the transplanted population.15 Specifically, the risk of ischaemic heart disease associated with diabetes, arterial hypertension or dyslipidaemia was significantly greater in the transplant population in comparison with the population as a whole according to this scoring system.
Proteinuria represents a first-order risk factor for graft survival and mortality from ongoing lesions caused by kidney grafts and endothelial dysfunction, as observational studies have shown.16-18 Furthermore, a large proportion of patients (60%) suffer a premature reduction in glomerular filtration rate (<60ml/min/1,73m2). This alteration is an importan cardiovascular risk factor, increasing mortality in this population.19-22 In a recent cohort study of TX patients, it was observed that an early (third month) combination of mild albuminuria (100-1000mg/day) plus renal graft dysfunction (30-60ml/min/1,73m2) significantly increased the risk of graft loss and death, possibly because of the combined effect of both alterations.23 Left ventricular hypertrophy is very frequent in these patients. 60% of TX patients show such an alteration, where poorer functioning of the kidney graft and the non-use of ACE inhibitors constitute independent risk factors for maintenance of this alteration.24 Inflammation can play a crucial role in the development of atheromatous vascular lesions. Elevated levels of C-reactive proteins - a biomarker for the inflammatory process related to atheromatosis in kidney patients - are associated independently with mortality, something which may represent a very useful predictor for these patients.25,26 In line with this, a cross-sectional study in uraemic patients with type 1 diabetes revealed an increase in proinflammatory cytokines, chemokines, cell adhesion molecules and nuclear factor kB (NFkB) in the inferior epigastric artery obtained at the moment of TX.27 Undoubtedly, this supports the idea of the potential role of proinflammatory molecules in the development of CVD in these patients.
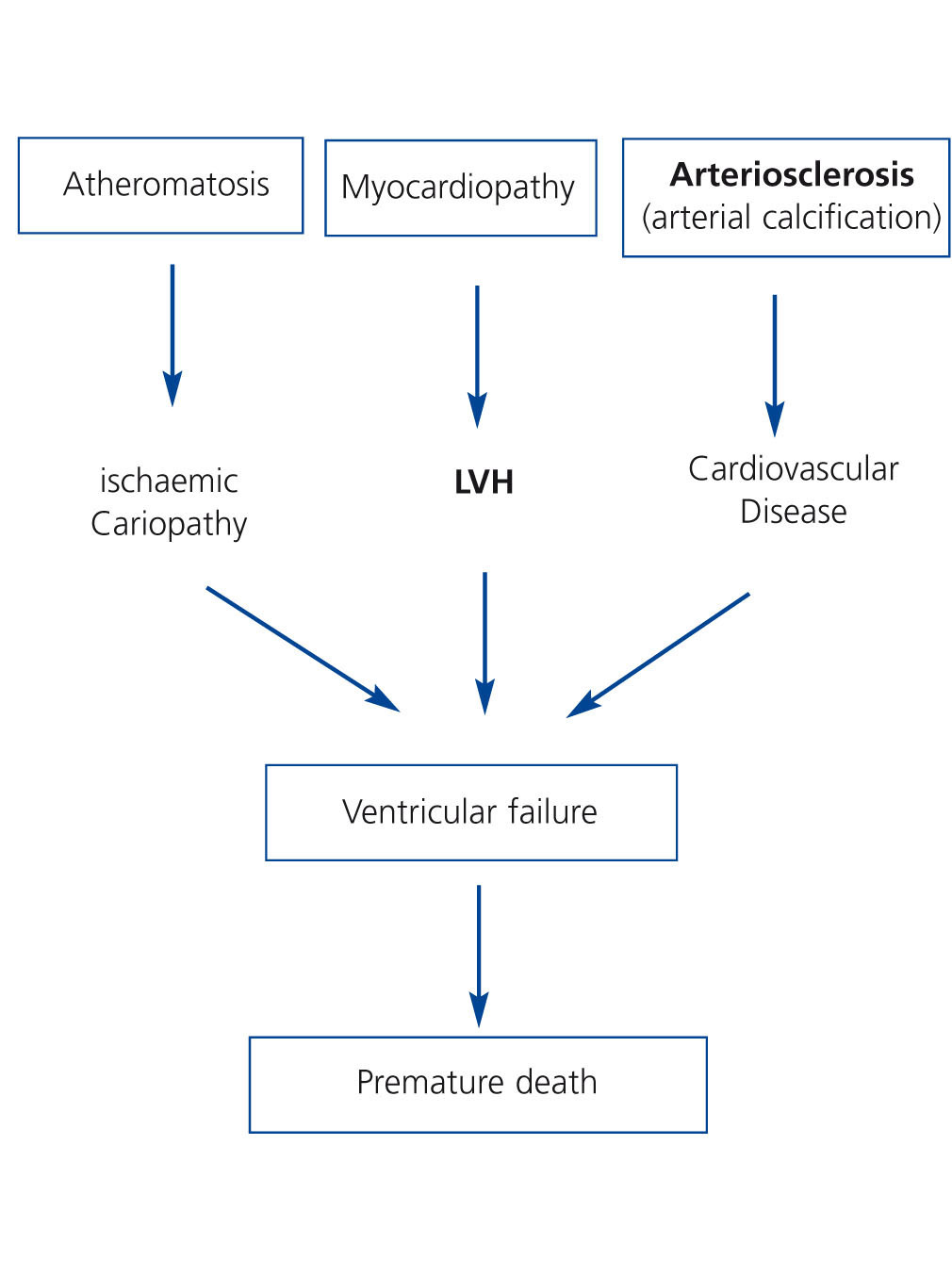
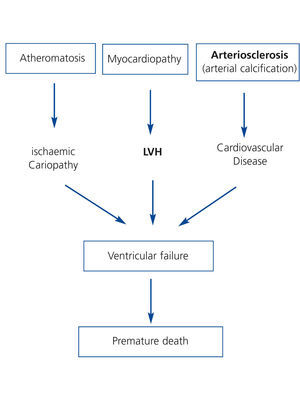
Pathogenically, these risk factors converge in 3 interrelated processes (Figure 3). First, a process of accelerated atheromatosis leading to ischaemic heart disease. Second, a phenomenon of anomalous cardiac remodelling, leading to left ventricular growth (concentric or eccentric) and ventricular dysfunction. Lastly, calcification of the middle arterial layers or arteriosclerosis, a process common in chronic kidney disease that tends not to revert after TX and which increases comorbidity. The final consequence is ventricular failure with a fall in cardiac output and premature death of these patients during follow-up. Indeed, ischaemic heart disease is highly prevalent after TX. The cumulative incidence of acute myocardial infarction in the first three years following TX is 11%, a fact which increases by 2.6 the risk of death post-TX.28 Furthermore, patients with left ventricular hypertrophy or vascular calcification prior to TX who were assessed by means of simple abdominal X-ray have higher mortality rates than those without these cardiac or vascular alterations, irrespective of other cardiovascular risk factors.29,30
At the same time, post-TX infections by immunomodulators such as the cytomegalovirus (CMV), herpes virus, Epstein-Barr virus, hepatotropic viruses or human immunodeficiency virus can alter the expression of inflammatory mediators and cytokines, increasing susceptibility to contracting serious infections. This phenomenon can also increase the risk of chronic graft dysfunction and the appearance of tumours and other metabolic or vascular illnesses that ultimately lead to a decrease in patient survival. In this respect, infection by CMV has been related with ischaemic peripheral artery disease, particularly when associated with other cardiovascular risk factors, such as smoking.31
Seropositivity for Epstein-Barr virus represents a first-order risk factor for the development of post-TX lymphomas, principally in young patients receiving induction with monoclonal antilymphocyte antibodies (OKT3) or thymoglobulin.32 In an American observational study that recruited 89 485 subjects, seronegativity for CMV or Epstein-Barr virus, as well as having received induction with antilymphocyte antibodies or anti-CD25, significantly increased the risk of post-TX lymphoproliferative processes, which in turn confers seventeen and twenty-five times more risk of death and graft loss, respectively, compared with those who did not develop a lymphoproliferative disorder.33
Lastly, there is a greater incidence of tumours in the transplant population in comparison with the general population and immunosuppression and certain viral infections play a decisive pathogenic role in their appearance.34 A report by the American register that gathered more than 175 000 patients demonstrated that the standardised incidence of cancer in the transplant population was twice that of the population in general and this included neoplasias that were both related and unrelated with infectious processes.35
3. THERAPEUTIC STRATEGIES THAT MAY MINIMISE MORTALITY
In this context, we should ask ourselves how to prevent these high rates of vascular, infectious and tumour comorbidity in TX patients in the era of modern immunosuppression. Given the high prevalence of metabolic and vascular disorders in this population, control of blood pressure, dyslipidaemia, alterations in carbohydrate metabolism and possibly the administration of antiaggregants could minimise comorbidity in these patients and, in theory, improve their survival rate. Pharmacological blocking of the renin-angiotensin system reduces blood pressure and decreases proteinuria and ventricular mass. This may translate into an improvement in survival. Observational studies have shown that the use of these drugs is associated with a reduction in mortality, by using propensity analysis and marginal structural models to avoid confounding by indication.36-38 In addition, a controlled clinical trial with TX patients demonstrated that the use of fluvastatin was associated with a reduction in LDL cholesterol levels and cardiovascular events compared with those receiving a placebo.39 That said, the use of cardioprotective and renoprotective drugs such as statins, beta blockers or aspirin is highly variable and relatively scarce during the first year post-TX in these patients, as multicentre observational studies have shown.40,41 This fact may explain, in part, the increased mortality rate when both traditional and non-traditional cardiovascular risk factors come together.
The type of immunosuppression and its intensity can contribute to elevated cardiovascular, infectious or tumour-related mortality following TX. Given the negative effects of immunosuppressive treatment, individualisation of immunosuppression may be one of the strategies that improve TX outcomes in terms of survival.42 Withdrawal of steriods or avoiding their use is associated with a slight increase in the acute rejection rate, but there is a notable improvement in the cardiovascular risk factor and the incidence of lipid disorders and carbohydrate metabolism. There is no doubt that this can contribute to decreasing the risk of mortality, as controlled and observational studies have shown.43-46 Cyclosporin (CsA) produces more hypertension and hyperkalemia than tacrolimus. In fact, conversion of CsA to tacrolimus improves these disorders, which translates into a reduction of the Framingham risk score in patients receving tacrolimus both in induction treatment and reconversion patterns.47
Tacrolimus is more diabetogenic than CsA,48 but lines of treatment involving minimalisation of steroids and the individualisation of calcineurin-inhibiting drugs could decrease the risk of post-TX diabetes, especially in patients with a greater predisposition to develop this condition. Controlled clinical trials will provide an answer to these questions.
Given the antiproliferative properties of anti-mTOR (mammalian target of rapamycin) drugs, sirolimus and everolimus can revert atheromatous vascular lesions and reduce left ventricular mass, as has been shown in the animal model or in longitudinal studies of TX patients.49,50 In this respect, a controlled, randomised study demonstrated that administration of everolimus plus a reduced dose of CsA significantly reduced ventricular mass compared with conventional doses of CsA, where treatment with everolimus represented an independent factor associated with regression of left ventricular mass.51 Conversion from a calcineurin inhibitors to anti-mTOR inhibitors is also associated with an improvement in renal function.52 In theory, this can also contribute to decreasing morbidity and mortality in these patients.
In the area of tumour pathology, the conversion of CsA to sirolimus has the ability to halt the progresson of Kaposi's sarcoma, a tumour associated with infection by herpesvirus 8 in TX patients.53 Controlled, randomised studies of patients at risk of developing non-melanocytic skin cancer due to previously suffering skin neoplasms have demonstrated that using sirolimus significantly reduces the risk of appearance of a second neoplasia when compared with continuing use of a calcineurin inhibitor.54,55
The use of non-nephrotoxic drugs such as belatacept may also help to minimise cardiovascular risk in this population. Controlled studies have shown that use of this drug is associated in the short and medium term with a lower incidence of post-TX diabetes and that it improves the lipid profile and optimises renal function in comparison with CsA, not to mention its immunological power.56,58
Lastly, other measures, such as infectious disease prophylaxis, especially use of valganciclovir against CMV, reduction or modulation of immunosuppression, periodical screening for neoplasms and the use of anti-mTOR in patients at risk of skin neoplasia or viral diseases can contribute to reducing infectious and tumour-related mortality post-TX, although more controlled studies are needed to confirm this.
4. PREDICTION OF MORTALITY AND ISCHAEMIC HEART DISEASE
Predicting mortality and comorbidity can be crucial in determining the most timely therapeutic measures and decreasing mortality and comorbidity in these patients. Therefore, for TX patients is absolutely necessary to employ prognosis indices that include comorbidity risk factors as well as secondary survival measures in order to estimate survival with greater precision with a view to making more accurate therapeutic decisions.
But, how is comorbidity quantified and what are prognostic indices for?. At an individual level, any risk variable (predictive) or secondary variable can determine prognosis or the outcome of a given clinical entity. However, predictive capability can be optimised by using multiple predictive variables or secondary measurements grouped into what we call comorbidity prognosis indices.
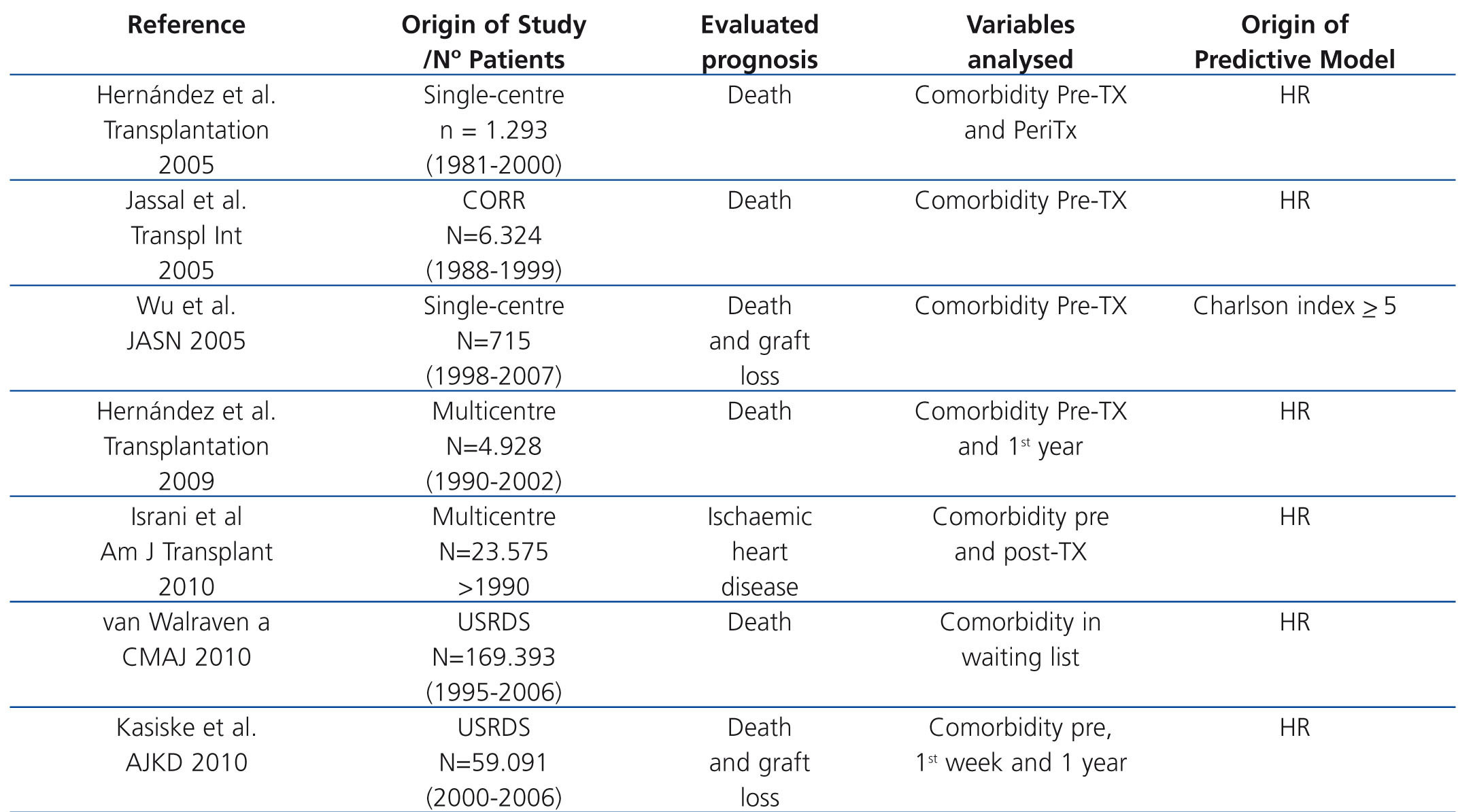
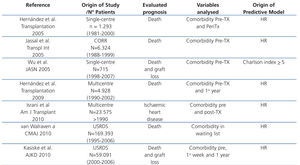
Recent years have seen the creation of different prognostic indices for mortality and comorbidity in TX patients. Some, such as the Charlson index,59 have been based on risk indices that apply to the population in general. Others have been based on pre-TX and post-TX variables originating with multicentre registers or single-centre studies (table 1). All predict the risk of death or comorbidity (principally ischaemic heart disease) with a high level of agreement and all have been validated both internally and externally with other populations. Let us briefly look at some examples that focus on mortality and ischaemic heart disease. Using the database of the Canadian TX Register, a comparison was made of the ability to predict mortality of 4 prognositic indices validated using the population as a whole and a uremic population, which revealed the Charlson index to be the most useful for predicting the final outcome for these patients60. Nevertheless, a high number of patients did not show significant comorbidity at the time of the TX and they did not include conditions inherent to the TX itself, which may render the results questionable. A later cohort study involving 715 patients who had transplants between 1998 and 2003 demonstrated that Charlson's index was in fact a good clinical tool for assessing morbidity. Patients with a score >5 had a greater risk of death and graft loss than those with a score <5, but once again, they did not use factors inherent to TX in order to predict the prognosis.61 In a single-centre cohort study, a mortality index was created and validated based on the statistical weight (beta coefficient) of all the pre-TX and peri-TX (during hospitalization) variables significantly associated with survival in the model population. From this, a summative scale was created that divided risk into terciles. These risk terciles included a combination of classic risk factors and perioperative factors inherent in TX (age, classic risk factors, ventricular hypertrophy, vascular calcification, obesity, diabetes, time on dialysis graft function, first or second TX and presence of delayed renal graft function), in such a way that, as risk increased, survival significant decreased.62 Similarly, using a database of American renal patients, a death-risk score has been created for three groups of patients: patients on waiting lists, patients with deceased-donor TX and patients with live-donor TX.63 Following randomisation of the sample into two subpopulations (model population and validation population), a score was obtained for each variable which in the Cox multivariate analysis is associated with mortality in the model population. In this manner, a summative score was obtained for each patient that was exponentially related with mortality at 5 years. Although this index shows comparative data for different modes of substitute treatment, it does not take into account inherent factors relating to the progress of TX. In fact,the majority of the prognostic indices used in TX only consider baseline (initial) risk factors and do not include emergent progress-related risk factors. Based on this premise, using the database of the Spanish Chronic Graft Nephropathy Group, which included 4928 patients receiving transplants in four different years (1990, 1994, 1998 and 2002) a predictive mortality index was developed based on a combination of basal risk factors and post-TX progress-related factors64. Beta coefficients of the variables in the population model associated in the Cox analysis with mortality were used to create a summative risk scale which was divided into quartiles. This scale was used to calculate the probability of death in the first three years of follow-up according to the following formula: P(death) = 1- 0.993964837exp(score points/100). The probability of death increased from <1% for the lowest quartile (score 40) to >5% in the highest quartile (score 200) and this estimated probability of mortality was similar to the mortality observed in the two subpopulations studied (model and validation) with acceptable discrimination (C-index 0.75 and 074). Following a similar design, a risk scale has been created for the development of ischaemic heart disease immediately post-TX, at one week from TX and after the first year of post-TX progress based on those variables associated with the primary event during the periods studied in the multivariate analysis.65 In addition, an exponential relationship was thus obtained between the summative risk and the probability of a cardiac ischaemic event.
KEY CONCEPTS
1. Death of patients continues to be the second highest cause of renal graft loss, despite improvements in clinical managment of these patients, especially the population with a longer life span.
2. CVD represents the most frequent cause of post-TX mortality in the era of modern immunosuppression, followed by infectious and tumour-related processes.
3. There are pathogenic interaction and mechanisms in common between cardiovascular entities, infectious and tumor-related processes which can increase mortality in these patients.
4. Risk factors emerging during post-TX progress may perpetuate high post-TX mortality, despite the optimisation in clinical management of this population as well as new immunosuppressants.
5. The rare use of cardio and renoprotective medication with these patients may explain, in part, this elevated post-TX mortality.
6. Individualisation of immunosuppression could improve the prognosis for these patients.
7. There are some very useful tools to predict mortality in this population and as a result, to establish therapeutic strategies aimed at improving outcomes for these patients.
Acknowledgements
This study was financed in part by the Andalusian Regional Ministry of Health (PI-0499/2009) and by the Spanish Ministry of Science and Innovation (Instituto de Salud Carlos III, FIS PI10/01020), REDINREN RD12/0021/0015.
Conflict of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Table 1. Predictive models of mortality and comorbidity in kidney transplant patients
Figure 1. Potential interaction between risk factors, comorbidity and mortality following renal transplantation.
Figure 2. Risk factors for post-kidney transplant cardiovascular disease
Figure 3. Potential pathogenic mechanisms of cardiovascular disease in post-kidney transplant patients