Introducción: El fracaso renal agudo (FRA) en el mieloma múltiple (MM) se presenta entre el 12-20% de los casos y la supervivencia de estos pacientes depende de la recuperación de la función renal. El 75% de los pacientes dependientes de diálisis no recuperan la función renal y su supervivencia media en situación de tratamiento sustitutivo es inferior al año. La nefropatía por cilindros es la causa más frecuente de fracaso renal y acontece en más del 55% de los casos, y en el 75% de aquellos que requieren diálisis. Para facilitar la recuperación de la función renal es imprescindible la disminución rápida de los niveles en sangre de cadenas ligeras. Una medida coadyuvante al tratamiento específico de la enfermedad ha sido la reducción de estas cadenas ligeras con plasmaféresis, sin que se haya demostrado claramente su eficacia, por lo que se ha propuesto el uso de hemodiálisis largas con filtros de alto poro (HCO), consiguiendo una tasa de recuperación superior al 60%. Presentamos la evolución en seis casos de pacientes con mieloma y fracaso renal agudo que fueron tratados con dichos filtros HCO, las complicaciones con este tipo de hemodiálisis y revisamos los pros y los contras de esta técnica. Metodología: Seis pacientes diagnosticados de MM y FRA con necesidad de diálisis y niveles circulantes de cadena ligera por encima de 500 mg/l fueron tratados con hemodiálisis de 8 horas con filtro HCO. Al comienzo y al final de cada sesión se medían las cadenas ligeras séricas por nefelometría, así como otros parámetros. Al mismo tiempo los pacientes fueron tratados con quimioterapia según protocolos. Resultados: A tres hombres y tres mujeres diagnosticados de MM y FRA, con inicio de los síntomas muy variable, desde 7 días a más de un año, se les realizó 90 sesiones de hemodiálisis largas con filtros HCO con un rango de entre 6 y 40 sesiones. El porcentaje de reducción de las cadenas ligeras desde el inicio del tratamiento hasta su finalización fue el 65% de media, excepto en un paciente, que fue del 12,6%. La media del porcentaje de reducción de la cadena ligera por sesión fue de 54,98 ± 17,27%. En el 28% de las sesiones se registró alguna complicación. El 48% de las complicaciones se debieron a la coagulación del sistema. No hubo grandes cambios en los niveles de albúmina prediálisis, calcio, fósforo y magnesio, aunque en algún caso se registraron valores disminuidos que no comportaron relevancia clínica. En tres pacientes la función renal se recuperó y permanecen vivos e independientes de la diálisis. En los casos biopsiados y que recuperaron función renal, la nefropatía por cilindros fue pura. Los pacientes que tardaron más en ser diagnosticados fueron los pacientes que no recuperaron función renal, y cuando se les efectuó biopsia el diagnóstico fue de nefropatía por cilindros más enfermedad por depósitos. Conclusión: En nuestra experiencia, la hemodiálisis larga con filtros HCO es una alternativa razonable en el FRA causado por nefropatía por cilindros, alcanzando en nuestros casos una tasa de recuperación del 50%. En la recuperación influyeron: el tiempo transcurrido desde el inicio de los síntomas al diagnóstico de mieloma, los hallazgos histológicos, la rapidez de instauración del tratamiento quimioterápico y su respuesta y la eficacia en la extracción de cadenas ligeras. En cualquier caso, son necesarios nuevos estudios con nuevos agentes quimioterápicos y las nuevas técnicas de extracción directa de cadenas ligeras.
Introduction: Acute renal failure (ARF) occurs in 12%-20% of all multiple myeloma (MM) cases, and the survival of these patients depends on renal function recovery. Renal function is not recovered in 75% of dialysis-dependent patients, and their mean survival with replacement therapy is less than one year. Renal tubular disease is the most frequent cause of renal failure. It is present in more than 55% of renal failure cases and in 75% of those requiring dialysis. Rapid reduction of free light chain levels in the blood is necessary in order to recover renal function. One coadjuvant measure in treating the disease is reducing light chain levels with plasmapheresis, but its efficacy has not yet been clearly proven. Our proposal was therefore to use extended haemodialysis sessions with high cut-off dialysers (HCO-HD), obtaining a recovery rate of more than 60%. We present the progress of 6 patients with myeloma and acute renal failure who were treated with HCO-HD and the complications associated with using this type of haemodialysis. Then, we review the pros and cons of this technique. Method: Six patients diagnosed with MM and ARF requiring dialysis and with serum free light chain levels above 500mg/l were treated with 8-hour haemodialysis sessions with an HCO-HD filter. Before and after each session, serum free light chain levels were measured by nephelometry; other parameters were recorded as well. At the same time, patients underwent chemotherapy according to protocols. Results: The symptom onset times of the 3 men and 3 women diagnosed with MM and ARF were highly variable, from 7 days to more than 1 year. We performed 90 extended sessions with HCO-HD filters, and each patient underwent between 6 and 40 sessions. Free light chain levels decreased by a mean of 65% between treatment onset and completion, except in one patient who experienced a 12.6% reduction. The mean percentage of reduction of light chain levels per session was 54.98%±17.27%. A complication occurred during 28% of the sessions. Of these complications, 48% were due to system coagulation. There were no major changes in pre-dialysis albumin, calcium, phosphorous or magnesium levels, although lower values that were not clinically relevant were recorded in one case. Renal function was recovered in 3 patients, they are alive and dialysis-free. In biopsied cases that recovered renal function, the specimen showed tubular nephropathy only. Those patients who took longer to be diagnosed did not recover their renal function, and when biopsied, they were diagnosed with renal tubular disease and light chain deposition disease. Conclusion: We found extended haemodialysis with HCO-HD filters to be a reasonable alternative in ARF caused by renal tubular disease, and achieved a recovery rate of 50% in our cases. Function recovery was influenced by the elapsed time between symptom onset and myeloma diagnosis, histological findings, promptness of starting chemotherapy, response to chemotherapy, and effectiveness of light chain extraction. In any case, further studies are needed to examine new chemotherapy agents and new direct free light chain removal techniques.
INTRODUCTION
Multiple myeloma (MM) is a plasma cell neoplasm that causes acute renal failure (ARF) in 12%-20% of cases, whether as an initial manifestation of the myeloma or during the course of evolution of the disease following diagnosis.1 The survival of these patients will depend on whether or not they recover renal function, not only due to the complications derived from the renal failure itself, but also from the reduced possibility of access to more effective treatments.2,3 Renal function is not recovered in 75% of dialysis-dependent patients,4-7 and their mean survival on renal replacement therapy is less than 1 year.8
Cast nephropathy is the most common cause of ARF and plays a role in over 55% of cases, and 75% of those that require dialysis.9,10 The formation of casts in the distal tubules, caused by the deposition of light chains and Tamm-Horsfall protein, is the main cause of renal failure in these patients11-14; as such, it is essential to rapidly reduce blood levels of free light chains in order to facilitate the recovery of renal function.15
Chemotherapy treatments for MM have improved greatly in the last decade, and regimens involving bortezomib, melphalan, thalidomide, and lenalidomide have improved the prognosis of these patients.16 One coadjuvant therapy for this disease has been reducing free light chain levels using extracorporeal purifying techniques. Plasmapheresis has been used for many years to reduce the circulating levels of light chains, although the efficacy of this treatment in recovering renal function has not been clearly demonstrated.17 This has caused researchers to search for new techniques that can effectively remove light chains and improve the recovery rate of renal function. To this end, Hutchison et al, using high cut-off (HCO) dialysers and long haemodialysis sessions (8 hours), have achieved good results in these patients, with recovery rates over 60%.18
We present the evolution of six patients with myeloma and acute renal failure that were treated with these HCO filters, as well as the complications that arise with this type of haemodialysis, and review the pros and cons of this technique.
MATERIAL AND METHOD
Six patients diagnosed with MM and ARF requiring dialysis and with serum light chain levels >500mg/l were treated with haemodialysis using HCO 1100® (1m2) or Theralite® (2.1m2) high cut-off filters made from polyaryl ether sulfone/polyvinyl pyrrolidone (Gambro Dyalisatorem, Henchingen, Germany).19 These filters are designed to increase the permeability of substances smaller than 60kD. The only difference between them is the greater surface area of the membrane, which increases the efficacy in removing light chains and produces a greater loss of albumin. We used Theralite® (2.1m2) filters when they were commercially available. The haemodialysis sessions involved standard haemodialysis monitors, lasted for 8 hours, involved a blood flow of 200ml/min-250ml/min, and had an ultrapure dialysate flow rate of 500ml/min. Sodium heparin was applied at 3000IU to start with, and 500IU at each hour. At the end of each session, 20% albumin was administered (100cc) along with monosodium phosphate (10ml) and magnesium sulphate (10ml), following the protocol set out by Hutchison et al.20 The goal was to perform 5 sessions on consecutive days, followed by sessions on alternating days until renal function was recovered or until light chain levels fell below 500mg/l. Ultrafiltration was programmed according to the clinical needs of each patient.
Before and after each session, mean free light chain levels were measured in terms of mg/l using nephelometry (FREELITE®; The Binding Site, Birmingham, UK),21 and creatinine, albumin, phosphorous, calcium, and magnesium were measured before each session. These measurements were also taken after each session in the first patient. In cases requiring a renal biopsy, an analysis was performed using a light microscope and immunofluorescence with standard techniques, including the detection of anti-kappa and lambda light chain antibodies by immunofluorescence. We also used a Congo red stain test.
At the same time, patients were treated with chemotherapy regimens according to the protocols of the haematology department.
RESULTS
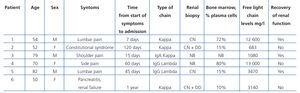
Three men and three women were admitted to the nephrology department for ARF of an unknown cause and were finally diagnosed with MM. The characteristics of the patients are summarised in Table 1. Three patients were younger than 60 years, and the other three were older than 70. The initial symptoms were bone pain in four cases, one patient sought treatment for constitutional syndrome, and the sixth patient was admitted under the diagnosis of unconfirmed pancreatitis and renal failure. The start of symptoms varied greatly, between 7 days and over a year (6th patient). In this patient, a review of the clinical history revealed that a monoclonal gammopathy and bone infarcts had been detected one year before, requiring health care, but with an incomplete analysis. The patients that took the longest time to be diagnosed were those that did not recover renal function.
Three patients had kappa light chain myeloma, two had lambda IgG myeloma, and one had IgA kappa myeloma. The bone marrow was infiltrated to a varying degree by plasma cells of an anomalous phenotype that are characteristic of the disease. Four patients underwent a renal biopsy: two were diagnosed with cast nephropathy and the other two were diagnosed with cast nephropathy and light chain deposition disease. Immunofluorescence was positive in glomerular samples for light chains in two of the deposition disease cases, and in the tubules and cylinders of all four cases. Interstitial damage was mild, and tubular reactivity with marked regenerative changes was similar in all cases. None of the Congo red stain tests resulted positive. One patient refused the biopsy (case 3), and the health of another (case 4) negated that possibility. Proteinuria ranged between 6680mg/dl and 570mg/dl, with no correlation observed with serum free light chain levels or histological findings.
The number of days of hospitalisation, days to diagnosis, days to start of HCO-HD, as well as the number of days elapsed between hospitalisation and the start of chemotherapy are shown in Table 2. The first three patients were treated with vincristine, adriamycin, and dexamethasone, and the last three were given bortezomib and melphalan and/or prednisone, according to the protocols of the haematology department.
It total, the six patients underwent 90 sessions of haemodialysis, with a range of 6-40 sessions each. The HCO-HD was interrupted due to: a) renal function recovery in three cases (cases 1, 3, and 5); b) in case 2, the start of HCO-HD was delayed several days since the patient had a large inguinal haematoma following catheterisation of the femoral vein, and a posteriori analysis showed that light chain levels had fallen below 500mg/l; c) in case 4, treatment was interrupted upon finding that the patient’s light chain levels remained above 700mg/l and anuria continued even after 7 sessions of haemodialysis; and d) in case 6, treatment was discontinued after 40 sessions of HCO-HD and three cycles of bortezomib and prednisone in the absence of a response, with light chains remaining at 2760mg/l and persistent oliguria.
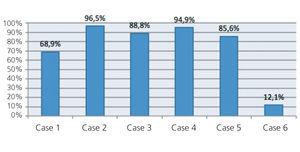
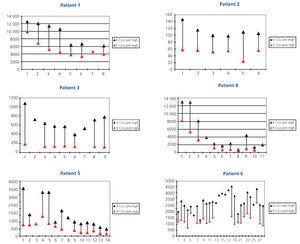
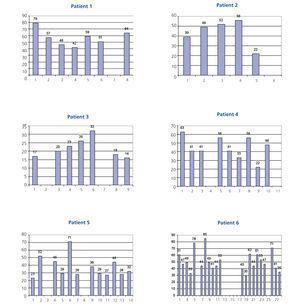
The percentage reduction in light chain levels from the start of treatment to the end of treatment in each patient is summarised in Figure 1, and was very high in all patients except for patient 6, who did not respond to chemotherapy treatment, with a small reduction in light chain levels (12.6%). In the other five patients, levels decreased by over 65%. In 56 sessions in which pre and post-HD light chain levels were measured, the mean percentage reduction in light chain levels was 54.98%±17.27% (Figure 2 and Figure 3). There were no differences in the reduction of lambda and kappa light chains (kappa: 53.52%±18.6% vs lambda 57.82%±14.2%), or between results when using 1.1m2 HCO or 2.2m2 Theralite® filters (56%±19% vs 53%±15%, respectively).
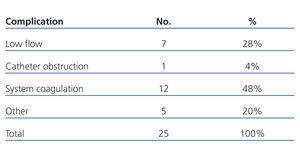
In 25 haemodialysis sessions (28%), several complications/events were registered. These are discussed in Table 3. On 12 occasions (48%), the complications were due to system coagulations (8 episodes in patient 6), requiring a suspension of the session on three occasions. Reduced flow and catheter obstruction were the causes of 8 other episodes (32%). Other complications were irrelevant.
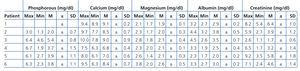
There were no major changes in predialysis albumin levels when using this method, although patients that were treated using the 2.1m2 Theralite® filter had a greater decrease in albumin, requiring increased doses after dialysis (cases 5 and 6). Although calcium, phosphorous, and magnesium levels were slightly reduced in several cases, this change had no clinical significance (Table 4). Other complications in the evolution of our study patients were: two infections (one resulted in a positive blood culture for Escherichia coli, the other one testing negative) and one stroke with complete recovery.
In three patients, renal function was recovered (cases 1, 3, and 5), and these patients remain alive and free of dialysis. Case 1 received a bone marrow transplant and has had favourable evolution for three and a half years; case 3 has been monitored for two years 8 months, and case 5 for 8 months. Of the three cases that did not recover renal function, one patient died 4 months after starting haemodialysis; in the other two cases, the patients are still alive, although they continue to depend on dialysis (case 2: 2 years, 10 months; case 6: 4 months). In the two cases that did not recover renal function and had undergone a renal biopsy, cast nephropathy was accompanied by deposition disease, whereas in the two cases that underwent a biopsy and recovered renal function, the cast nephropathy was the sole diagnosis.
DISCUSSION
Based on our experience, patients with MM and ARF with cast nephropathy can recover renal function through combined treatment with chemotherapy and long haemodialysis sessions with HCO filters, as occurred in at least 50% of the patients in our small study.
Recent studies have demonstrated that a rapid decrease in free light chain levels with chemotherapy and extracorporeal techniques is associated with recovered renal function.15,22-24 Several studies have evaluated the efficacy of plasmapheresis in the treatment of myeloma-related renal failure. The study by Zucchelli et al,23 and more importantly, the study by Johnson24 demonstrated a better patient evolution in those cases that were treated with plasmapheresis vs those that were not; however, the small sample size, the fact that not all patients had cast nephropathy, and the use of different dialysis methods significantly limited the conclusions made in this study. Afterwards, Clark17 published a randomised, controlled study involving 97 patients, the largest study available in the literature. In this study, plasmapheresis did not achieve superior results in terms of death, dependence on dialysis, and glomerular filtration rate (<30%ml/min/1.73m2). However, Leung15 found that plasmapheresis was effective at recovering renal function (mean time: 2 months) if the renal damage was due to cast nephropathy and the level of free light chains decreased by at least 50%. In summary, the efficacy of plasmapheresis in myeloma kidney is uncertain at best, and has yet to be fully upheld as a valid treatment after the publication of only a small number of studies with few patients. The reason for this is that plasmapheresis only purifies the intravascular space (17%) and not at the quantity necessary to provide a benefit to many patients, since the reduction in light chain levels depends primarily on the level of production.5 Furthermore, plasmapheresis cannot be sustained for a very long time.
Since 2007, the use of long haemodialysis sessions with membranes that are permeable to proteins has been proposed in order to increase the extraction rate of light chains up to 90% during three weeks.20 In this study, HCO filters (Gambro) with a cut-off of 45kD were superior to four different high-flux dialysers (10kD) and two super-flux dialysers (20kD) in clearing light chains (22.5-31kD). In this same study, the HCO filter was tested in 11 patients with myeloma and requiring haemodialysis who underwent different chemotherapies. Of the 11 patients diagnosed with cast nephropathy based on a renal biopsy, 5 underwent dialysis on a daily basis for one week and afterwards on alternating days; 3 recovering renal function. In the 2 patients that did not recover renal function, infections forced an interruption of the chemotherapy provided, with a consequent rebound in light chain levels. Later, these same authors reported their experience with 19 patients with renal biopsies showing cast nephropathy, 13 of which recovered renal function. Of the 6 patients in which chemotherapy had to be suspended, only one recovered renal function, which appears to suggest that the extraction of light chains using this method is only useful when accompanied by a response to chemotherapy.18
Following the first experience by Hutchison et al, we started treating patients with MM and ARF from cast nephropathy with this method of haemodialysis, with similar results to those from previous authors. In our six patients, the extraction of light chains in each long haemodialysis session with HCO filters reduced light chain levels by 53%-57%, similar to the results reported by Hutchison. At the end of the treatment, our patients had experienced decreases of over 70% over the initial values, except for patient number 6, who, despite the fact that haemodialysis was effective in reducing light chain levels, did not respond to chemotherapy and never recovered renal function, as occurred in Hutchison’s patients.18
We did not observe severe complications during haemodialysis sessions in our study, and with the replacement of lost albumin, calcium, phosphates, and magnesium, we reached acceptable levels in all these parameters. The use of 2.1m2 filters did result in a greater loss of albumin, which required increased doses to replenish albumin levels after dialysis sessions, using a double filter.25 The two infectious complications produced were not significant, and did not require halting the chemotherapy regimen.
With regard to the recovery of renal function, our results are similar to Hutchison’s18: approximately 60% of patients recovered renal function using this technique.22 Several factors influenced these results: 1) the time elapsed between the start of symptoms and the diagnosis of myeloma. In our study, the three patients that did not recover renal function were those that had suffered symptoms for the longest amount of time (60 days, 120 days, and 1 year). 2) Histological findings: the 2 patients that underwent a renal biopsy and did not recover renal function had deposition disease, in addition to cast nephropathy. This situation involves a poor prognosis and short life expectancy.26 3) The speed with which chemotherapy treatment is started and the response to this treatment. 4) The efficacy in removing light chains in haemodialysis using high cut-off filters. The possibility of recovery was 80% in patients whose light chain levels decreased by 60% after three weeks of treatment.18
There is still no proven evidence that this technique clearly contributes to improve survival in patients with MM and cast nephropathy, or that this type of treatment is superior to plasmapheresis, which has not demonstrated its effectiveness either. Currently, a randomised controlled study (EuLITE) is underway, involving 90 newly diagnosed patients with myeloma caused by cast nephropathy and dialysis-dependent renal failure. All patients will receive bortezomib and will be randomised to treatment with standard haemodialysis or HCO filter haemodialysis. The primary variable is independence from dialysis after three months of treatment.27 If results are favourable for this technique, it will then be compared with plasmapheresis.
Additionally, one limitation for the use of this technique is its high economic cost. Grima28 recently presented a cost-effectiveness model comparing the treatment of myeloma kidney with HCO filters vs standard haemodialysis. This study found that the treatment of myeloma kidney with long haemodialysis sessions using HCO filters could substantially improve renal recovery in patients with multiple myeloma, compared to the standard haemodialysis. This results in an improved life expectancy and overall savings, without the need for chronic haemodialysis. The model predicts a mean survival of 19.92 months in patients treated with standard haemodialysis vs 33.9 months using the new technique, with a total savings of 6000 pounds sterling per patient.
In conclusion, the poor prognosis of patients with MM and ARF due to cast nephropathy requiring dialysis, the relationship between reduced light chain levels and the recovery of renal function suggest the potential benefit of early chemotherapy combined with a method for the extra-corporeal removal of light chains that is effective and can be maintained over time. Long haemodialysis sessions with HCO filters are a reasonable option, although new studies involving new chemotherapy agents and new direct extraction methods of light chains are needed.
The authors declare potential conflicts of interest:
The author(s) are paid for conferences.
The author(s) receive a travel bursary/allowance.
Table 1. Characteristics of the six patients diagnosed with multiple myeloma
Table 2. Days to diagnosis and to treatment with HCO, chemotherapy used in the six patients diagnosed with multiple myeloma
Table 3. Complications in HCO haemodialysis
Table 4. Maximum, minimum, mean, and standard deviation of phosphorous, calcium, magnesium, albumin, and creatinine values before haemodialysis sessions
Figure 1. Percentage reduction in free light chain levels in the six patients treated with HCO haemodialysis at the end of treatment
Figure 2. Serum free light chain levels before and after haemodialysis in the six patients
Figure 3. Percentage reduction in serum free light chains in the haemodialysis sessions of all six patients