In 2023, the Spanish Society of Nephrology's Glomerular Diseases Study Group (GLOSEN) published a consensus document containing the most pertinent information and clinical recommendations for the diagnosis, treatment, and follow-up of lupus nephritis (LN). GLANCE is a project that emerged from the need to evaluate the extent of knowledge and application of these GLOSEN recommendations in routine clinical practice for the management of LN. To achieve this, an online self-administered survey was conducted to gather opinions on the recommendations and assess their impact on clinical practice. Fifty-one Spanish nephrologists (n = 51) with more than three years of experience in managing LN and handling more than one LN patient per month, participated in the survey. All participants demonstrated a comprehensive understanding and high overall acceptance of the GLOSEN recommendations. However, discrepancies were noted regarding criteria for partial remission and relapse, as well as treatment goals during the initial months of progression, underscoring the need for a more detailed consensus. Other findings highlighted the limited number of nephrologists using specific scales to assess extrarenal manifestations and the tendency to extend immunosuppressive treatments beyond the recommended 3–5-year period outlined in the document. This emphasizes the necessity for further studies on the discontinuation of these drugs and their association with the risk of relapse in LN.
En 2023 se publicó el documento de consenso del Grupo de Estudio de Enfermedades Glomerulares de la Sociedad Española de Nefrología (GLOSEN), que recogía la información y las recomendaciones clínicas más relevantes para el diagnóstico, tratamiento y seguimiento de la nefritis lúpica (NL). GLANCE es un proyecto que surge de la necesidad de evaluar el alcance obtenido en términos de conocimiento y aplicación de estas recomendaciones GLOSEN en la práctica clínica habitual del manejo de la NL. Para ello, se llevó a cabo una encuesta, en línea y autoadministrada, para recoger opinión sobre las recomendaciones y conocer su impacto en la práctica clínica. Participaron nefrólogos españoles (n = 51) con más de tres años de experiencia en el manejo de la NL que atendieran a más de un paciente con NL al mes. La totalidad de participantes demostró un amplio conocimiento y una elevada aceptación global de las recomendaciones GLOSEN. No obstante, se observaron discrepancias en relación con los criterios de remisión parcial y recaídas, así como con los objetivos de tratamiento durante los primeros meses de evolución, lo que pone en evidencia la necesidad de consensos más elaborados. Otros resultados destacaron el limitado número de nefrólogos que utilizan escalas específicas para evaluar las manifestaciones extrarrenales, y la tendencia a prolongar los tratamientos inmunosupresores más allá del período de 3−5 años tal y como se recomienda en el documento, resaltando la necesidad de más estudios sobre la retirada de estos fármacos y su asociación con el riesgo de recaídas en la NL.
Systemic lupus erythematosus (SLE) is a chronic systemic autoimmune disease, in which autoantibodies generate circulating immunocomplexes that deposit in various organs. The appearance of these deposits in the renal glomeruli leads to lupus nephritis (LN), which is considered one of the most severe organ manifestations.1 The prevalence of LN among SLE patients is approximately 40%, but ranges from 12% to 69% in different populations2 and its mortality is 5–8 times higher than in the general population.3–5
In 2023 the Glomerular Diseases Working Group of the Spanish Society of Nephrology (GLOSEN) developed a consensus document to address the increasing complexity of treating LN.6 The GLOSEN document is an update of a previous consensus, published in 2012,7 and brings together a series of practical but rigorous recommendations based on the best available evidence to unify criteria and optimize the diagnostic, therapeutic and follow-up approach to LN. The online GLOSEN recommendations, hosted by the Revista de Nefrología, registered 61,333 visits between February 2023 and January 2025. Also, according to ResearchGate data, other investigators cited the publication 8 times during this period.
A few months after its publication, the GLANCE project (GLOSEN consensus survey for the diagnosis and treatment of LN) was developed, arising from the need to assess its scope in terms of its popularity and applicability. This evaluation is crucial to measure the effectiveness of the implementation of the proposed recommendations, to identify areas for improvement and to ensure that clinical practitioners follow the guidelines, with the aim of detecting discordances or other obstacles to implementation.
The objectives of our study were to measure the degree of awareness of the GLOSEN consensus document among nephrologists in Spain, to evaluate the impact and implementation of this document in routine clinical practice and to analyze the feasibility of incorporating these recommendations for improving the diagnosis and treatment of LN.
Material and methodsStudy designThis is a descriptive survey-based study, in which we systematically collected data on the recommendations contained in the GLOSEN Consensus Document (Appendix 1) by means of a structured, self-administered, online questionnaire following a quantitative analysis methodology. The survey was designed to assess both the degree of understanding of the recommendations and their impact on clinical practice. We used closed questions with single or multiple answers, which allowed us to obtain quantitative results on the level of knowledge and the frequency of application of each of the recommendations included in the questionnaire. Likewise, we carefully considered the order of the questions to avoid a response to previous questions conditioning subsequent answers; thus, we listed questions about the reality of clinical practice before those evaluating GLOSEN knowledge.
Between November-December 2023, a scientific committee composed of 3 GLOSEN consensus authors supervised the conceptualization of the survey. After review and validation, we opened the questionnaire for participation from March 20 to May 21, 2024, and completed the final statistical analysis of the results in June 2024.
We invited specialist participants in the GLOSEN Group, the Spanish Society of Nephrology (SENEFRO), which endorses this study, and other regional nephrology societies.
Justification of the methodologyWe chose to conduct a survey because it makes it possible to easily access a broad group of nephrologists, to collect data systematically, and efficiently to evaluate the aggregated data. In addition, it allows professionals to express their perspectives anonymously without bias, facilitating a better understanding of the real impact of recommendations on the diagnosis and treatment of LN.
Population and sampleThe survey was aimed at nephrologists in Spain with direct and relevant experience in the management of LN. Selection questions ruled out the participation of other medical specialties or resident interns, nephrologists with less than 3 years of experience, those who referred patients after the first visit, those who were not involved in the management of LN and/or those who managed a number of patients with LN equal to or less than one per month.
The nephrologists were invited by GLOSEN to participate in the questionnaire through a link sent by e-mail, guaranteeing the confidentiality of the responses. Although the sample was not systematic or randomized, we invited specialists all over the country, to achieve homogeneous geographical representation and include hospitals of various levels, thus giving greater validity to the results. Instructions were given for completing the survey and assistance was provided to resolve doubts.
All the participants selected (n = 51) met the inclusion criteria and there was no inconsistency in any of their responses. Of 119 experts who initiated the survey 68 were excluded (Fig. S1 of the Supplementary material), mainly due to an incomplete survey (n = 41).
Data analysisDescriptive statistical analysis was performed for quantitative questions. For ordinal/nominal variables, absolute frequency (number) and relative frequency (percentage of responses for each category relative to total responses [%]) were calculated.
We collected the data in a database and analyzed it using the IBM SPSS Statistics Base 27 software program.
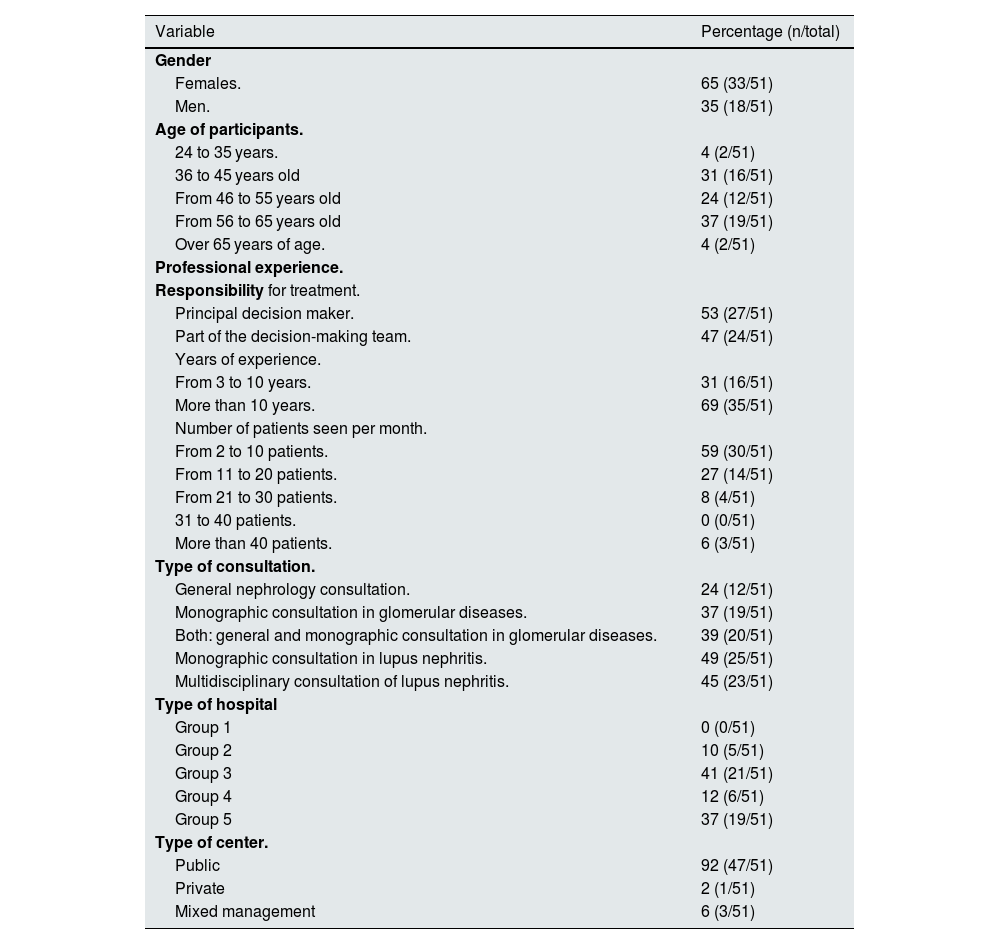
ResultsDemographic data and participant profileThe demographic characteristics and profile of the participants are represented in Table 1. Some 43% (n = 51) of the invited nephrologists fully completed the questionnaire. The expert panel consisted mostly of women (65%), older than 35 years and with more than 10 years of experience in LN. Fifty-three percent of the nephrologists considered themselves the main decision-makers in the treatment of LN, attending a minimum of 2–10 patients per month and, for the most part, in public hospitals (92%), where 37% of the experts attended their patients in a glomerular disease monographic consultation. In addition, 49% had a monographic unit for the management of LN and 45% had a multidisciplinary management of the disease with Rheumatology and/or Internal Medicine units.
Demographic characteristics and profile of participants.
| Variable | Percentage (n/total) |
|---|---|
| Gender | |
| Females. | 65 (33/51) |
| Men. | 35 (18/51) |
| Age of participants. | |
| 24 to 35 years. | 4 (2/51) |
| 36 to 45 years old | 31 (16/51) |
| From 46 to 55 years old | 24 (12/51) |
| From 56 to 65 years old | 37 (19/51) |
| Over 65 years of age. | 4 (2/51) |
| Professional experience. | |
| Responsibility for treatment. | |
| Principal decision maker. | 53 (27/51) |
| Part of the decision-making team. | 47 (24/51) |
| Years of experience. | |
| From 3 to 10 years. | 31 (16/51) |
| More than 10 years. | 69 (35/51) |
| Number of patients seen per month. | |
| From 2 to 10 patients. | 59 (30/51) |
| From 11 to 20 patients. | 27 (14/51) |
| From 21 to 30 patients. | 8 (4/51) |
| 31 to 40 patients. | 0 (0/51) |
| More than 40 patients. | 6 (3/51) |
| Type of consultation. | |
| General nephrology consultation. | 24 (12/51) |
| Monographic consultation in glomerular diseases. | 37 (19/51) |
| Both: general and monographic consultation in glomerular diseases. | 39 (20/51) |
| Monographic consultation in lupus nephritis. | 49 (25/51) |
| Multidisciplinary consultation of lupus nephritis. | 45 (23/51) |
| Type of hospital | |
| Group 1 | 0 (0/51) |
| Group 2 | 10 (5/51) |
| Group 3 | 41 (21/51) |
| Group 4 | 12 (6/51) |
| Group 5 | 37 (19/51) |
| Type of center. | |
| Public | 92 (47/51) |
| Private | 2 (1/51) |
| Mixed management | 6 (3/51) |
All participants were aware of the contents of the GLOSEN document (82% belonged to GLOSEN). It is considered a standard clinical practice guideline for 92% of respondents and highly valued (considered necessary, easy to understand and practical); it is used more frequently than other guidelines such as European Alliance of Associations for Rheumatology (EULAR)8,9 or Kidney Disease Improving Global Outcomes (KDIGO).10
Impact of GLOSEN recommendations on current clinical practiceDiagnosis and follow-upAll respondents agreed with the need to perform a renal biopsy for a better diagnosis and classification of LN, unless contraindicated. When considering the criteria for biopsy, 86% adhered to the indicated parameters of proteinuria of >0.5 g/24 h (Fig. S2 of the Supplementary material).
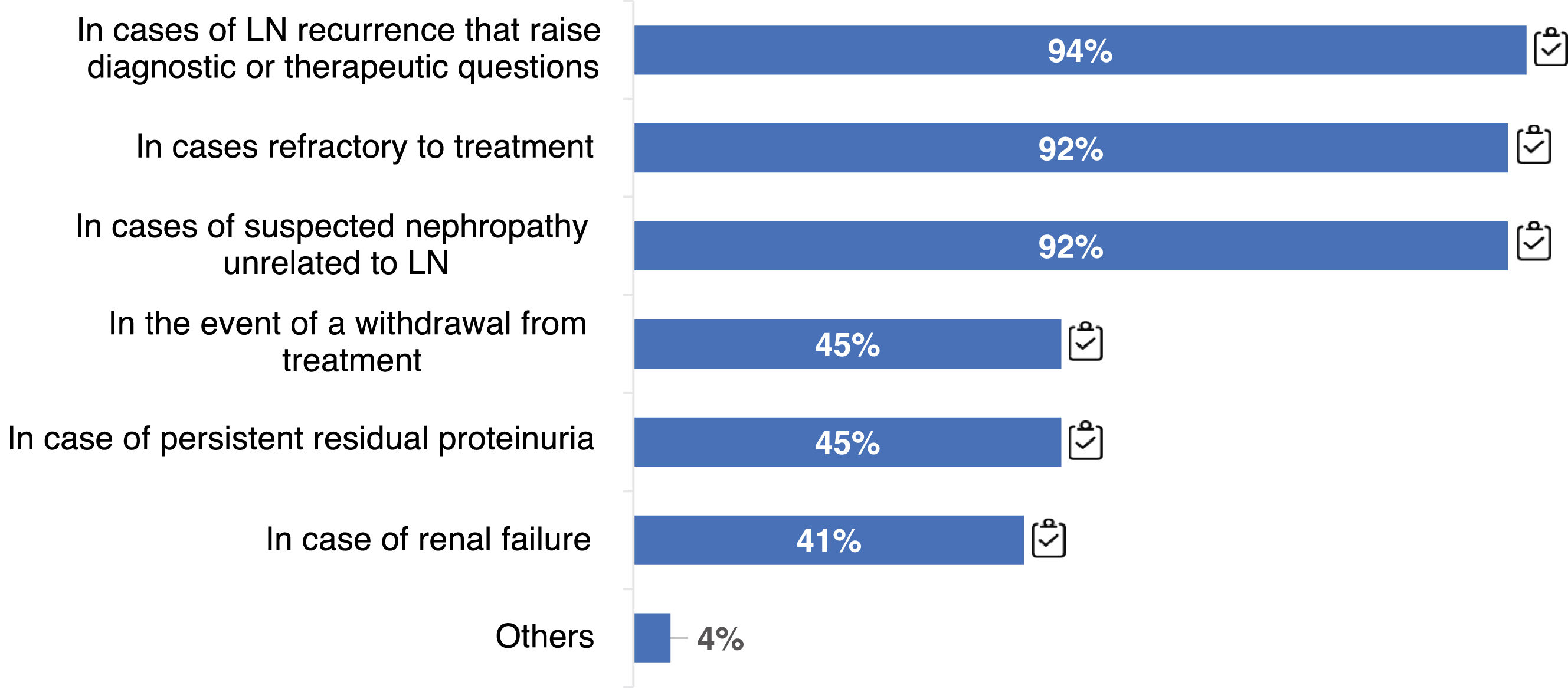
100% of respondents indicated renal re-biopsy at some point during disease follow-up, either for relapses, refractoriness to treatment, or in cases of suspected nephropathy other than LN (Fig. 1). Re-biopsy could also help in deciding to withdraw immunosuppressive (IS) treatment (45%).
Virtually all participants (98%) assess extrarenal manifestations during LN follow-up, although 63% do not use any specific scale. Only 31% use the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI).
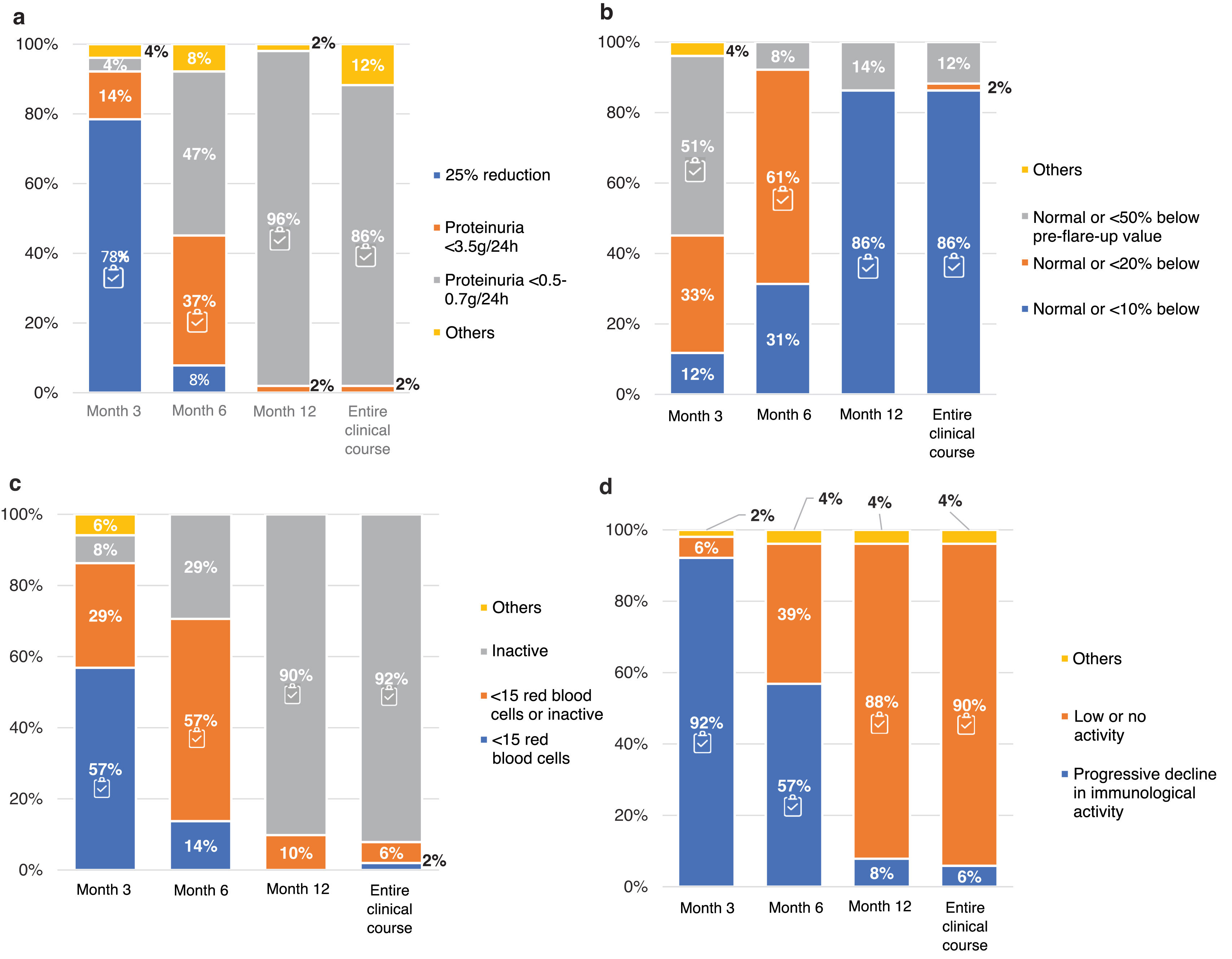
The proteinuria, estimated glomerular filtration rate (eGFR), urinary sediment and immunological activity targets at 3, 6 and 12 months, and throughout the clinical course, are in Fig. 2. There was consensus in trying to achieve normalization of proteinuria (< 0.5−0.7 g/24 h), urinary sediment (inactive) and immune activity (low or absent) values at 12 months of treatment and throughout the clinical course of the disease. However, respondents were more demanding than indicated in the guidelines regarding the evolution of eGFR in the first months of treatment (normal or ≤50% lower than the prebreak value). In relation to intermediate objectives 78% of the participants set as a goal a 25% reduction of the initial proteinuria value before the third month of treatment.
LN treatment targets. Question asked: In your opinion, what targets should be achieved for: (a) proteinuria; (b) eGFR; (c) urinary sediment; (d) immune activity at different time points: at 3, 6 and 12 months and throughout the clinical course?
The results are expressed as percentages (%).
eGFR: estimated glomerular filtration rate.
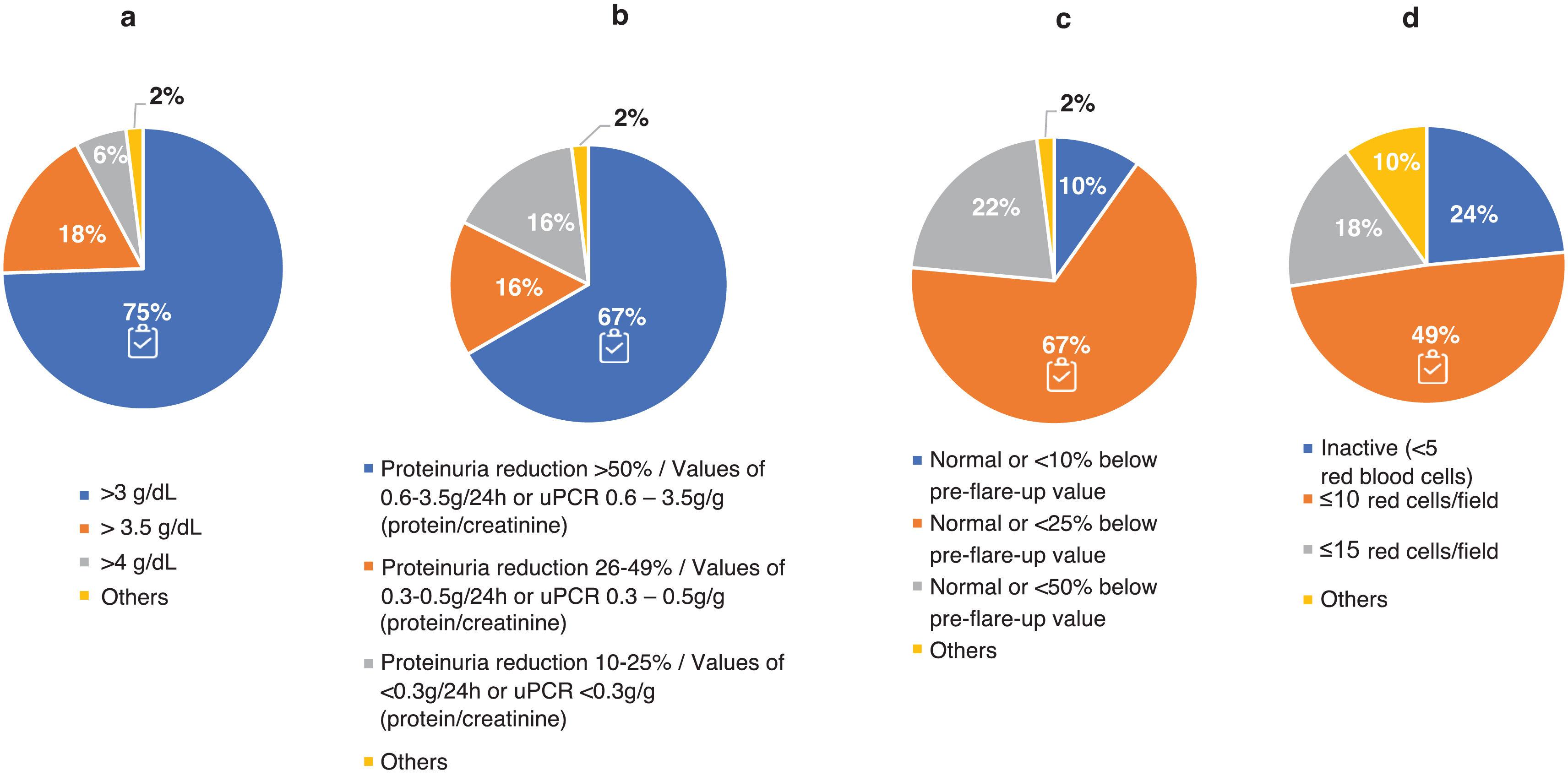
Adherence to the recommendations provided in the GLOSEN guidelines.Regarding the definition of complete response (CR), 88% closely followed the criteria defining this objective. However, there was more controversy in the definition of partial response (PR) (Fig. 3). The experts were mostly aligned with the guidelines regarding serum albumin concentration (75% agreed that it should be ≥3 g/dl) and eGFR (67% opted for a normal value or at least 25% lower than the prebreak value). Similarly, there were different levels of requirement for proteinuria, although 67% of nephrologists adhered to the GLOSEN consensus recommendation (≥50% reduction in proteinuria). Regarding hematuria 49% followed the GLOSEN recommendation (≤10 red blood cells/field [H/C]).
Criteria for the definition of partial response (PR) to treatment of LN. Question asked: In your opinion, by what values would you determine whether a patient of yours has achieved partial response (PR)? Think of a typical patient profile that you see in the office: (a) serum albumin concentration (g/dL); (b) reduction levels/range of proteinuria values (g/day; or g/g urine protein-to-creatinine ratio [uPCR]); (c) renal function (estimated glomerular filtration rate [eGFR]); and (d) reduction in hematuria (red cells/field).
The results are expressed as a percentage (%).
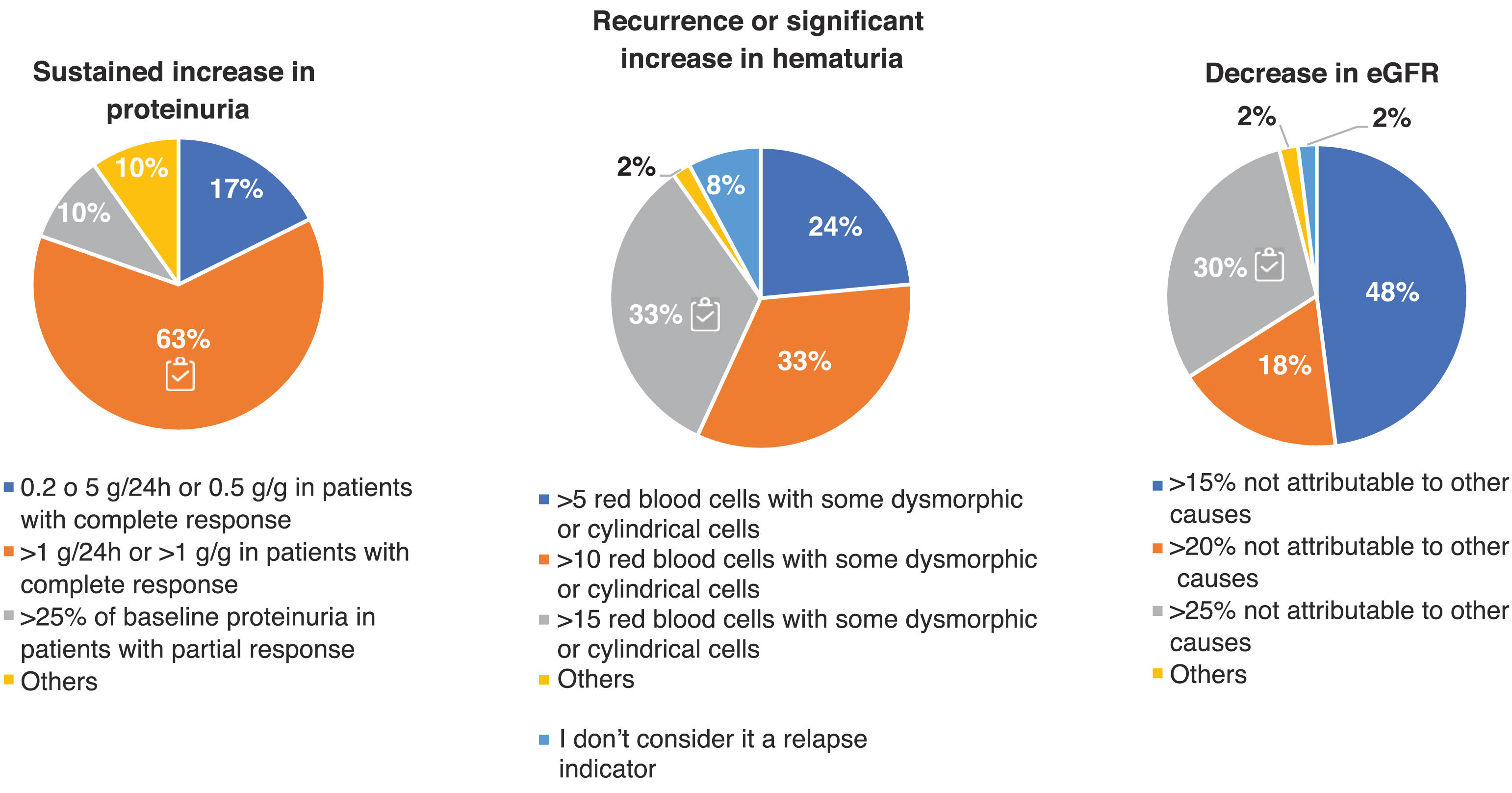
Adherence to the recommendations provided in the GLOSEN guideline.On the other hand, the criteria indicating relapse of LN were very disparate. In Fig. 4 we can observe how around only 30% of the experts agree with the consensus regarding the values of hematuria (>15 H/C) and decreased eGFR (≥25% not attributable to other causes).
Criteria for the definition of relapse in patients with LN. Questions asked: In your opinion, which of the following criteria and values do you consider indicative of relapse in clinical practice? For the definition of relapse, the evaluation criteria were: sustained increase in proteinuria (g/day or g/g), recurrence or significant increase in hematuria, and decrease in estimated glomerular filtration rate (eGFR). Results are expressed as percentages (%).
Adherence to the recommendations provided in the GLOSEN guideline.Although there is no specific recommendation in the GLOSEN consensus on what the frequency of reevaluation of the patient with LN should be, most agreed on monthly reevaluation during induction therapy (96%) and every 3 months during maintenance (86%). We observed a greater discrepancy regarding the re-evaluation of the disease after discontinuation of immunosuppressive treatment, the tendency being a frequency of every 6 months, while a third of the sample indicated every 3 months.
Pharmacological and non-pharmacological treatment of lupus nephritisAlmost total adherence to the GLOSEN consensus was found with respect to the guidelines for nonpharmacological (no smoking, controlled diet, aerobic exercise) and pharmacological (cardio- and nephroprotective drugs and hydroxychloroquine [HCQ] of LN) treatment.
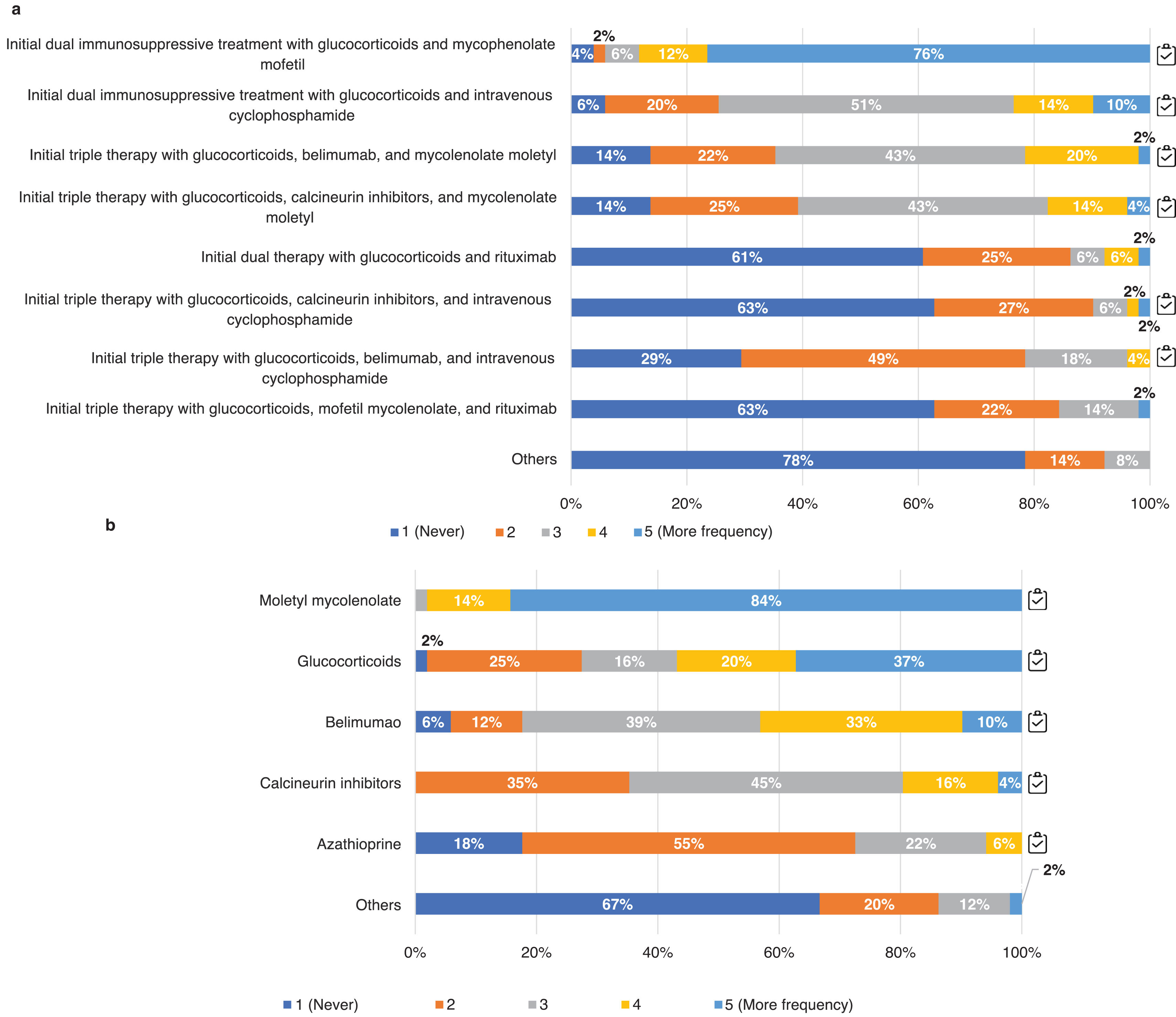
Respondents, according to their usual clinical practice, indicated the frequency of use of possible induction and maintenance treatment regimens for class iii and iv ± v LN (Fig. 5).
Pharmacological management of LN. Questions asked: a) Thinking of your usual clinical practice indicate the frequency of use of the following induction treatment regimens for class iii, iv LN with or without v; b) thinking of your usual clinical practice, indicate the frequency of use of the following immunosuppressive treatments for maintenance therapy of class iii, iv LN with or without v. We quantified the degree of agreement with a Likert scale from 1 to 5 (1: never; 2: rarely; 3: sometimes; 4: frequently; 5: most frequently).
The results are expressed as percentages (%).
Adherence to the recommendations provided in the GLOSEN guideline.There was a clear preference for initial dual versus triple immunosuppressive therapy in all classes, opting for the combination of glucocorticoids (GC) and mycophenolate mofetil (MMF; 88%) over cyclophosphamide (CYC; 24%). The use of CYC, mostly the euro-lupus regimen (low-dose CYC), is considered above all in cases of non-response to treatment or severe systemic manifestations (78%). Other patient profiles considered include non-adherence (66%) or a second LN flare (20%). Regarding triple therapy, if it was based on MMF and GC, the use of belimumab (BEL) or calcineurin inhibitors (CNI) was similar; however, when the regimen was based on CYC and GC, the use of BEL was preferred. For the maintenance phase most preferred MMF treatment, followed by GC and BEL for proliferative classes (Fig. 5b) and CNIs for class v.
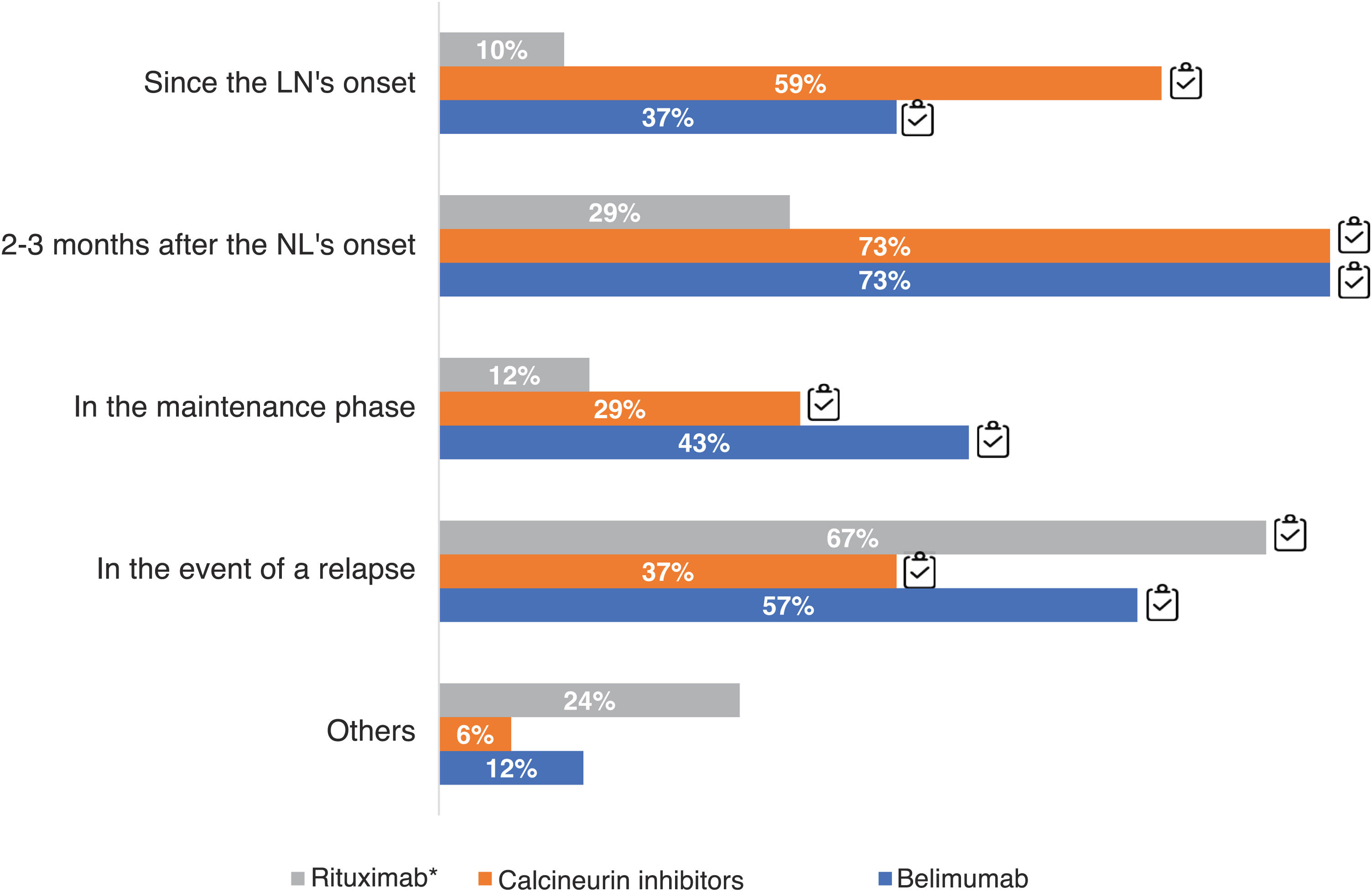
73% of the experts used CNI 2–3 months after the onset of LN and 59% from the onset, while the majority use of BEL was also 2–3 months after the onset of LN (73%) or upon relapse (57%). Only 37% considered the use of biologics at disease onset(Fig. 6).
Timing of therapeutic use of belimumab, calcineurin inhibitors and rituximab. Questions asked: when do you usually add belimumab; when do you usually add calcineurin inhibitors; when do you usually add rituximab? The results are expressed as percentages (%).
LN: lupus nephritis.
Adherence to the recommendations provided in the GLOSEN guideline.*n = 49 have responded that they use rituximab in their clinical practice.
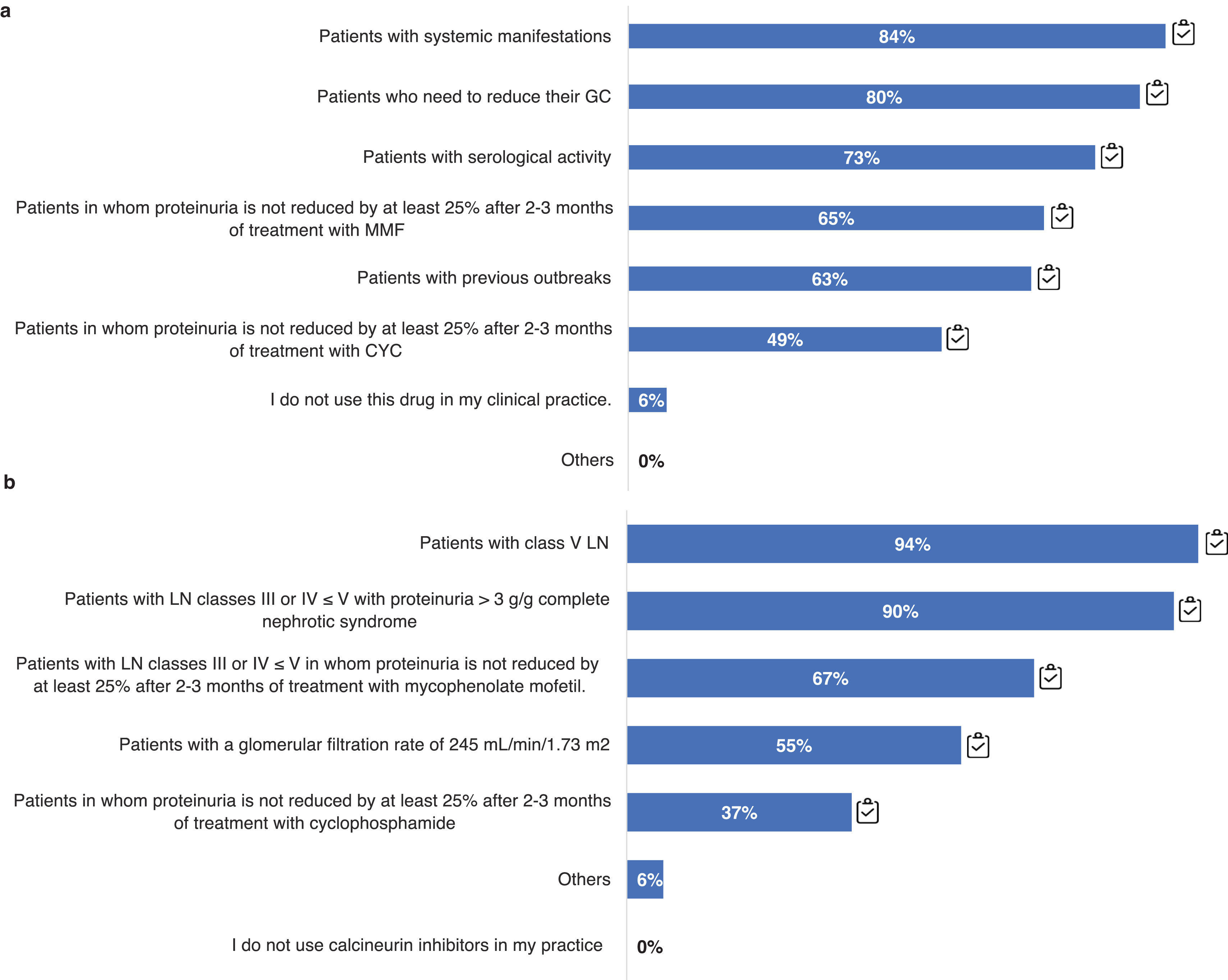
In relation to this point, we also analyzed the patient profiles in which the use of BEL and CNI is considered. The use of triple therapy from the start with BEL is widespread in patients with systemic manifestations (84%), or with the need to reduce corticosteroids (80%), but less so, for example, in patient profiles with previous outbreaks (63%) (Fig. 7a). Regarding the use of BEL in maintenance, most use it in patients with extrarenal manifestations (94%) or persistent serologic activity (82%), as well as in patients with frequent relapses (84%). Regarding the use of triple therapy with CNI it is mostly used in patient profiles with class V LN (>90%) and/or nephrotic syndrome (90%), and in those in whom proteinuria did not decrease more than 25% after 3 months of immunosuppressive treatment (67%) (Fig. 7b).
Therapeutic management of LN according to different patient profiles. Practical clinical question: a) Which patient profile uses belimumab in your clinical practice? b) Which patient profile uses calcineurin inhibitors in yoru clinical practice?
CYC: cyclophosphamide; GC: glucocorticoids; MMF: mycophenolate mofetil; LN: nephritis. Results are expressed as a percentage (96).
Adherence to the recommendations provided in the GLOSEN guidelines.The use of azathioprine was considered in patients intolerant to MMF, in pregnant women or with gestational desire.
With respect to rituximab, it was one of the drugs of choice for cases of refractory LN, non-responders to previous therapies or those who developed side effects with other treatments. In addition, 67% of respondents reported using it for disease relapses (Fig. 6).
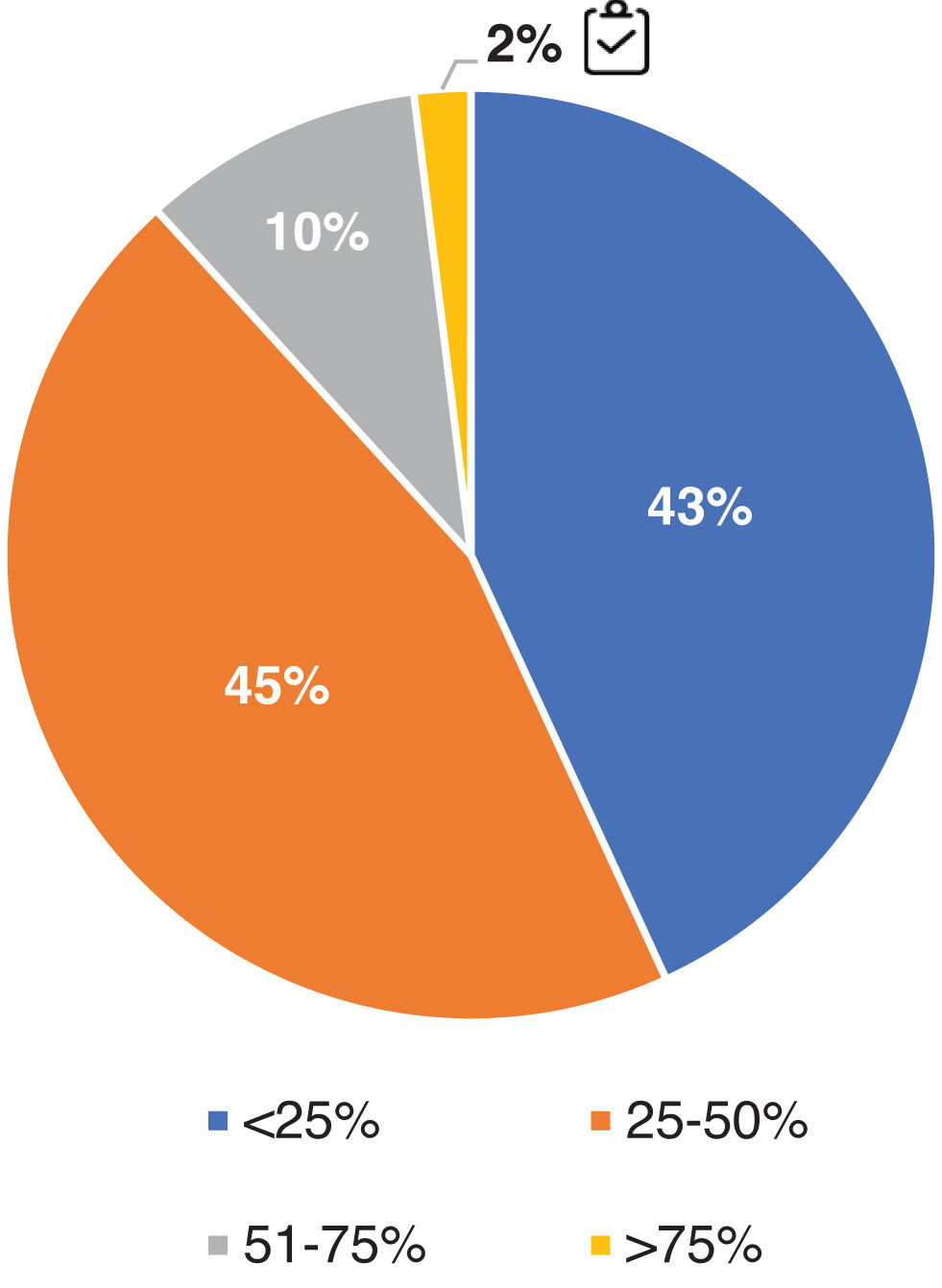
Withdrawal of glucocorticoids and immunosuppressive therapyFig. S3 in the Supplementary material reflects the panel's opinion on the dose of maintenance GC used in patients with LN. 92% follow the recommended dose of maintenance GC for LN (≤5 mg/day). Only 2% of experts completely discontinue immunosuppressive treatment (steroids included) in more than 75% of their patients, after a maintenance period of 3−5 years (Fig. 8).
Therapeutic management of LN with respect to discontinuation of immunosuppression. Question asked: in what percentage of your patients do you estimate that you have been able to completely discontinue immunosuppressive treatment (including steroids) after 3-5 years of maintenance therapy?
Results are expressed as a percentage (%).
Adherence to the recommendations provided in the GLOSEN guideline.This survey shows the impact of the GLOSEN consensus on clinical practice. The degree of variability and discrepancy in some results has proven that 3 important challenges persist: (a) definition of treatment and follow-up objectives: unify definitions of PR and relapse; whether to perform re-biopsies in follow-up; (b) use of combined therapeutic strategies vs. sequential therapies: dual or sequential therapy continues to be used more than triple therapy or combined strategy in all patient profiles; and c) discontinuation of long-term immunosuppressive treatment: yes or no, when? Only 2% of respondents discontinue IS treatment.
Defining treatment goals and follow-up in lupus nephritisThe panel's opinion on treatment goals for LN shows different levels of stringency, probably due to the variety of parameters included in the different guidelines. Proteinuria is currently the most widely used marker to assess treatment efficacy in LN.11,12 However, different techniques can be used for its determination, which would lead to a certain disparity between the possible methods of evaluation for this and other parameters. GLOSEN warns that the use of urine test strips, or quantifications by urine volume, should be avoided because they will provide inaccurate measurements.6 Regarding urinary sediment, automated techniques, for use in the quantification of red blood cells and leukocytes per field, have replaced traditional manual techniques, which are superior for the detection of casts and morphological abnormalities.13,14
The efficacy of treatment depends on whether the patient response is complete or partial. In all cases, the aim is to achieve complete response as quickly as possible to extend renal survival.6 It is very important to have a clear definition of treatment response and adherence by all healthcare professionals to homogenize decision-making on the therapeutic approach to LN. The definition of CR was clearer than that of PR. Although improvement or normalization of urinary sediment is not included in the EULAR8 or KDIGO10 guidelines, most respondents described hematuria as a sensitive marker of clinical activity, especially in cases of previous relapse and residual proteinuria.6
Between 10 and 50% of patients with LN present relapses.15–17 The differences between the parameters included in the different guidelines to define a relapse of LN are even greater than in the definitions of CR or PR.8,10 The disparity demonstrated by the participants on relapse indicators highlights the need to strengthen the criteria and values of proteinuria, hematuria and eGFR defining relapse for a more uniform implementation in clinical practice, as well as to ensure appropriate patient management. Although the risk of relapse is higher in the first years after the onset of LN, it can occur at any time, even after several years of inactivity. Therefore, ongoing periodic monitoring is recommended.6 Although GLOSEN does not specify the frequency of follow-up, a clear need for patient monitoring was shown during all phases, opting to schedule a regimen of visits every month or every 3 months, in the induction or maintenance therapeutic phases, respectively.
The usual clinical and analytical parameters are not sufficient to identify renal histological alterations in many cases of LN,6 so we used renal biopsy. This technique is key to determine the histological class of LN, establish a prognosis and plan treatment. According to the survey, there was a high level of agreement regarding the GLOSEN-recommended criteria6 for biopsy in patients with SLE: proteinuria > 0.5 g/24 h, active urine sediment, and/or rapid deterioration of renal function.
On the other hand, re-biopsy is still under debate because of its possible complications and its influence on patient management,18 with clinicians considering it unnecessary in cases with good progress and response.19 Although its implementation is progressing and has good adherence to guidelines/consensus, its use depends on the resources available in each hospital. Some experts prefer serological biomarkers (cytokines, growth factors and/or urine autoantibodies)20–23 as an alternative to rebiopsy.24
The survey results show that 45% of experts prefer re-biopsy before deciding to withdraw immunosuppressive treatment. One of the risks of withdrawal of immunosuppression is that LN may flare up and require reintroduction of treatment.25 A renal biopsy performed prior to withdrawal of immunosuppression may help this decision by assessing for the existence of activity lesions that may predict relapse.26 However, this is a controversial issue, as other authors advocate a gradual withdrawal of immunosuppression with careful assessment of analytical and serological data without the need for biopsy.27
The identification, assessment and follow-up of extrarenal clinical manifestations is fundamental in LN. However, most of the respondents do so without scale. This is a problem that may perhaps be due to the required consultation time and the lack of training of nephrologists and patients. Better training in this regard would help in the systematization of this assessment, in line with the GLOSEN consensus.
Use of triple therapyThe therapeutic algorithm of the GLOSEN document recommends selecting the therapeutic scheme according to the patient's clinical and analytical profile, from double (GC + MMF or CYC in Eurolupus guideline) or triple (GC + MMF + BEL or GC + MMF + CNI) initial immunosuppressive therapies. During the last few years the BLISS-LN,28 BEL and AURORA29 (voclosporin) clinical trials have illuminated multitarget or triple therapy of LN,30 changing the usual management of this disease. However, and as reflected in the results of this survey, the use of dual therapy is still the general preference in all patient profiles.
Regarding the patient profile candidate for BEL or CNI, in general there was a good overall agreement between the recommendations of the GLOSEN document and the opinion of the respondents: BEL was preferable for patients with extrarenal manifestations, persistent serological activity, need to reduce the dose of GC or relapse prevention; and CNI for patients with proteinuria >3 g/day or complete nephrotic syndrome.
Regarding maintenance therapy, respondents agree with GLOSEN about the use of MMF with low-dose GC as the mainstay of immunosuppressive treatment. Forty-three percent consider the use of BEL in this maintenance phase, which coincides with the demonstrated ability of this drug to reduce the risk of relapse and progressive loss of renal function.31
Despite the lack of international consensus on the definition and treatment of refractory LN,32 the experts followed the GLOSEN recommendations, mainly using rituximab. Importantly, despite reported evidence of rituximab efficacy in refractory disease,33,34 triple therapy with GC + MMF + rituximab failed to demonstrate superiority over dual therapy with GC + MMF.35 However, a sub-analysis of this study suggested that this was due to incomplete depletion of CD19+ lymphocytes.36 Other studies show that the response to rituximab remains subject to complete CD20 lymphocyte depletion.37
Another striking result of the survey is the use of rituximab as first-line treatment for relapsed LN, which is not in line with GLOSEN recommendations. One possible explanation is that respondents may have confused the terms relapse and refractoriness when answering the survey, since the drug is indicated in GLOSEN as first-line treatment for refractory LN. Another possibility is the unavailability of BEL as an officially accepted therapy for patients with LN and frequent relapse before 2021.
Withdrawal of immunosuppressive therapyThe GLOSEN document recommends withdrawal of maintenance immunosuppressive treatment (both GC and MMF) after at least 3–5 years of therapy, always with due flexibility and considering the possibility of relapse. However, the survey results reflect a significant tendency to maintain long-term use of immunosuppressive and steroids for fear of relapse, even respondents recognized the benefits for side effects of reducing their use. There are several clinical challenges in investigating the reasons for this low discontinuation of immunosuppression. This is a controversial and complex issue because national and international guidelines show discrepancies, resulting in a lack of clear guidance. This highlights the need for prospective studies on immunosuppressive withdrawal in LN and, no less important, the prospective validation of emerging serum and/or urinary biomarkers that reliably reveal the presence or absence of histological activity and that justify, in a non-invasive and safer way, the therapeutic decisions to maintain or withdraw IS treatment.
Many specialists are empirically in favor of maintaining lifelong immunosuppression, since abandoning immunosuppression could mean a flare-up of the disease and an increased risk of relapses. Studies such as MAINTAIN show that more than 50% of patients maintained some type of immunosuppression after 10 years.38 Other authors, on the other hand, advise maintaining or gradually reducing immunosuppression until discontinuation in patients with LN in complete or partial clinical remission, after the results of a renal biopsy.24 This new perspective of recent use of sparing therapies, not only of GC, but also of IS, could allow better long-term treatment management, contributing to decrease organ damage derived from prolonged IG use.30
This study has certain limitations. The participants included do not represent all geographical areas of Spain, and more than half of the nephrologists who initially responded to the survey were eventually excluded from the analyses, all of which could generate opinion and selection bias. Nevertheless, inclusion criteria were established to ensure that the respondents had direct and relevant experience in the management of LN, with the aim of obtaining well-founded and useful responses for the purpose of the study and seeking to maintain a balance between the breadth of participation and the quality of the information collected. Likewise, the voluntary and unpaid nature of participation meant that the final sample size depended on the availability and motivation of the professionals to complete the questionnaire. While it is true that the sample size did not allow for robust statistical sub-analyses, we did explore trends according to classificatory variables, such as years of professional experience or number of patients seen, without observing significant differences between groups.
There was no direct interaction among the participants, which limits the possibility of discussing and debating different points of view, and valuable information could be lost, so that, to mitigate this limitation, a scientific committee was responsible for designing and evaluating the whole process and interpreting the results obtained. However, the systematic and anonymous collection of data on professionals who frequently treat patients with LN gives strength to the results found.
Finally, the GLOSEN 2023 consensus is highly valued for its utility and clarity, although its impact is not expected to increase in the future. Its potential can still be maximized; its practicality in the clinic and ease of implementation of key messages aimed at specialists and managers, should be reinforced. Thus, according to those surveyed, the main barrier to implementation is resistance to change on the part of managers due to the cost of some treatments or techniques, so it would be advisable to discuss with them the benefits of applying these recommendations using objective cost-effectiveness and cost-utility data.
In this regard, practitioners should ensure that guidelines are easily applicable in clinical practice and that the consensus is updated according to expert feedback and therapeutic advances in LN. We propose that medical societies create summaries or infographics with key points and visual formats, and that seminars, workshops and clinical sessions be organized to facilitate their implementation.
In conclusion, the degree of variability and discrepancy in some survey results has highlighted aspects of LN pending a more consensual definition, such as the case of the criteria for partial remission and relapse, and the performance of re-biopsies throughout follow-up. Another aspect to highlight is the low percentage of nephrologists using specific scales to evaluate extrarenal manifestations. The experts surveyed agreed with the initial immunosuppressive treatment schemes (double or triple therapy) proposed by the GLOSEN document based on patient profile, although with a greater use of double therapy, mainly with GC + MMF, versus initial triple therapy (adding BEL or CNI depending on the clinical profile). Finally, another relevant finding is the low number of respondents who discontinue immunosuppressive medication after the 3−5 year maintenance period recommended by GLOSEN. This important discrepancy highlights the need for further studies on this poorly studied aspect of LN.
CRediT authorship contribution statementM.M., G.F.J and M.P. contributed to the conception, design, analysis and interpretation of the survey results, as well as scientific and intellectual input in the drafting and revision of the initial and revised manuscript. A.I.A, M.E, M.E, X.F, C.G.C, M.G, M.G, E. M, L.F.Q.P, and J.E.R.R contributed scientific and intellectual reviews in the drafting and revision of the manuscript. All authors approved the final accepted version of the manuscript for this publication.
Ethical considerationsThe questionnaire was completed voluntarily by professionals, maintaining the confidentiality and anonymity of the answers collected, in accordance with the ethical code of conduct of ESOMAR and AEDEMO, the General Data Protection Regulation and current national regulations.
This survey, being an anonymous opinion survey aimed exclusively at professionals and not involving patients, interventions, or collection of biological samples or sensitive data, does not require review by an ethics committee. This approach is consistent with previously published similar studies, where the privacy, confidentiality, and safety of participants are not compromised.
FundingThis work was funded by the pharmaceutical company GSK, which reviewed this publication without influencing the results of the GLANCE survey.
Spanish Society of Nephrology EndorsementThis project and its publication are endorsed by the SEN.
M.M. has received fees for consulting services, participation in committees, symposia, and other services as an expert. In addition, he has received grants/research support.
G.F.J. has received fees for consulting services, participation in committees, symposia and other services as an expert. In addition, he has received research grants/support.
A.I.A. has received fees for services for participation in committees, symposia and other services as an expert. In addition, he has received research grants/support.
M.E. has received fees for consulting services.
M.E. has received honoraria for consulting services.
X.F. has received honoraria for consulting services, participation in committees, symposia and other expert services.
C.G.C. has received fees for consulting services, participation in committees, symposia and other expert services. In addition, he has received research grants/support and is a member of the editorial board of several organizations.
M.G. has received honoraria for consulting services, participation in committees, symposia and other expert services. In addition, he has received research grants/support.
E.M. has received honoraria for consulting services, participation in committees, symposia and other services as an expert. In addition, he has received research grants/support from the Instituto de Salud Carlos III (ISCIII) (RD24/0004/0018).
L.F.Q.P. has received fees for consulting services, participation in committees, symposia and other services as an expert.
J.E.R.R. has no conflict of interest.
M.P. has received fees for consulting services, participation in committees, symposia and other services as an expert.
The authors of this paper would like to thank all the participants in the GLANCE survey, whose anonymity has been preserved at all times, and especially all the members of the GLOSEN working group, for being a reference in the study of glomerular disease and for their valuable contribution, which has made possible both the development of the questionnaire and the subsequent writing of this manuscript. Finally, we would like to thank Maria Ruart, PhD, Maria Coll, MSc, and Ana Fernández, MSc of Adelphi Targis SL for their contribution to the survey design, data collection, data analysis and interpretation, as well as the preparation and medical writing support for this manuscript.






![Criteria for the definition of partial response (PR) to treatment of LN. Question asked: In your opinion, by what values would you determine whether a patient of yours has achieved partial response (PR)? Think of a typical patient profile that you see in the office: (a) serum albumin concentration (g/dL); (b) reduction levels/range of proteinuria values (g/day; or g/g urine protein-to-creatinine ratio [uPCR]); (c) renal function (estimated glomerular filtration rate [eGFR]); and (d) reduction in hematuria (red cells/field). The results are expressed as a percentage (%). Adherence to the recommendations provided in the GLOSEN guideline. Criteria for the definition of partial response (PR) to treatment of LN. Question asked: In your opinion, by what values would you determine whether a patient of yours has achieved partial response (PR)? Think of a typical patient profile that you see in the office: (a) serum albumin concentration (g/dL); (b) reduction levels/range of proteinuria values (g/day; or g/g urine protein-to-creatinine ratio [uPCR]); (c) renal function (estimated glomerular filtration rate [eGFR]); and (d) reduction in hematuria (red cells/field). The results are expressed as a percentage (%). Adherence to the recommendations provided in the GLOSEN guideline.](https://static.elsevier.es/multimedia/20132514/0000004500000009/v1_202511241335/S2013251425001348/v1_202511241335/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w94GCRvdQBB6xyQjMrWMzrts=)








