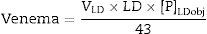The addition of phosphorus (P) to the dialysate (LD) in the form of enema Casen® is common practice in patients with hypophosphatemia. The estimation of the amount to be used and the identification of the problems that may occur are not well defined. As a result of our work we propose a practical approach of how to proceed to increase phosphate concentration in the hemodialysate. We present a reasoned formula to calculate how much enema has to be added and the problems that may arise.
La adición de fósforo (P) en el líquido de hemodiálisis (LD) mediante enema con fosfato de sodio (enema Casen®) se utiliza habitualmente en pacientes con hipofosforemia. El cálculo de la cantidad y los problemas que puede presentar no se describen en la literatura. Nuestro trabajo hace un abordaje práctico de cómo poner fósforo en LD con una fórmula razonada para calcular cuánto volumen de enema añadir en función del concentrado de diálisis utilizado y los problemas que pueden aparecer.
At haemodialysis (HD) units we find patients with intermittent or persistent hypophosphatemia (hypoP), which may be symptomatic. The frequency, the causes and the consequences of hypoP in chronic HD patients are not well known.1 During the past year, in our hemodialysis unit a 9.35% of serum phosphate values were less than 2.5mg/dl. HypoP may induce: rhabdomyolysis, haemolysis,2,3 leucocyte dysfunction, respiratory failure, impaired myocardial function, bone disease, etc.4,5 and in the elderly HD patient, hypoP has been associated with an increase in mortality.6
There are dialysates (LD) containing phosphate (P) for continuous acute dialysis techniques,7,8 but there are no commercial preparations for chronic HD. Phosphate can be added to the dialysis fluid in the form of Casen® enema, or other products with a high content of P,9 but no publication shows a practical approach on how to supplement it. Our goal is to describe how to calculate the volume of enema to be added to the acid dialysis concentrate to achieve a given concentration of P in the LD, the validation of this procedure, and the problems that may be encountered with this manoeuvre.
Theoretical basis to obtain the formulaFirst: How is P added to the LD? It is most common to use Casen® enema (Casen-Fleet, S.L. laboratories) containing 13.9g of anhydrous sodium dihydrogen phosphate and 3.2g of anhydrous disodium hydrogen phosphate. In Spain, there are presentations of 80, 140 and 250ml. Each ml contains 43mg of P.
Second: To what solution should we added? Since bicarbonate powder cartridges are the most commonly used, P should be added to the acid concentrate.
Third: How do I calculate how much enema to add?
- a.
What is the dilution acid to water that I use? The standard dilutions are 1:35 or 1:45. In the case of 1:45, every litre of acid result in 45l of LD.
- b.
What is the volume of the acid concentrate? In our case, we have: 3.5l bottles (Fresenius 5008®) and 5l bags (AK-200®). Therefore, using acid dilution of 1:45, 5l will generate 225l of LD (45×5) and using 3.5 l will end up in 157.5l (45×3.5).
- c.
What is the target P concentration in the LD ([P]LDobj)? If we want 1.5mg/dl, the amount of P required will be the result of multiplying [P]LDobj by the total volume previously calculated of LD (1.5×2250dl or 1.5×1575dl) which results in 3375 or 2362.5mg of P for the AK-200® and Fresenius® monitors, respectively.
- d.
What volume of enema contains such an amount of P? Since 1ml of enema contains 43mg of P, divide the amount of P by 43. If 3375 or 2362.5 are divided by 43, the result is that 78 and 55ml of enema need to added to the 5 and 3.5l acid containers, respectively.
- e.
The formula is:
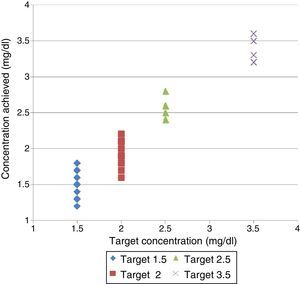
The monitors used were: Fresenius 5008® and Gambro AK-200®. The amount of enema required to achieve a [P]LDobj of 1.5, 2, 2.5, and 3.5mg/dl was calculated with 2 acid concentrates: 3.5l and bottles of 5l bags, with 1:45 dilution (Table 1).
Volume of enema needed to obtain the target P concentration in dialysis fluid and the results obtained.
| Target=1.5mg/dl | Target=2mg/dl | Target=2.5mg/dl | Target=3.5mg/dl | |||||
|---|---|---|---|---|---|---|---|---|
| AK® | 5008® | AK® | 5008® | AK® | 5008® | AK® | 5008® | |
| V (ml) | 78 | 55 | 105 | 73 | 130 | 92 | 183 | 128 |
| N | 20 | 14 | 17 | 4 | 4 | 4 | 3 | 4 |
| CC (mg/ml) | 1.59 | 1.55 | 1.96 | 1.96 | 2.53 | 2.55 | 3.53 | 3.2 |
| SD | 0.14 | 0.19 | 0.12 | 0.29 | 0.1 | 0.1 | 0.06 | 0.13 |
CC: mean concentration of phosphorus reached; SD: standard deviation; N: number of determinations made; V: added volume.
Bold is the obtained P concentration.
In AK-200®, we used another acid solution of 1l containing citrate, to generate a Ca 3.3mEq/l and K 2mEq/l with a [P]LDobj=1.5mg/dl. Using the same formula, with a 1:45 dilution, the volume of enema added was 15ml.
The concentration of P in LD ([P]LD) was measured 80 times in 2 separate situations:
- -
Sixty-five determinations in the LD of patients receiving P during their dialysis, with enema added to the acid concentrate due to hypophosphatemia (<2.5mg/dl), using a [P]LDobj=1.5 or 2mg/dl. All had a calcium concentration of 3mEq/l, except those dialysed with a LD containing citrate. The potassium concentrate was: 1.5mEq/l (in 39), 2mEq/l (in 13) and 3mEq/l (in 19).
- -
The remaining 15 were obtained from LD with [P]LDobj=2.5 or 3.5mg/dl. This LD was not used in patients.
The P was added to the acid concentrate before starting the monitor and it was shaken. The dialysis was prepared as usual. Twenty minutes after monitor indication that the LD was ready, samples were extracted. Any incidence during the preparation of the monitor, addition of P to the concentrate or sampling, was recorded.
Laboratory methodsPre-analytic preparation of the samples was not necessary because the LD lacks cells. P, Na, K, and Mg were determined using the Dimension EXL (Siemens) analyser. Direct colorimetric methods were used to determine P (phosphomolybdate method) and Mg; indirect potentiometry for Na, and K and direct potentiometry for ionic Ca in the Rapidlab 1265 (Siemens) gas analyser. To verify reproducibility, each sample was analysed with 2 identical machines.
Stability of the dilution was evaluated in 8 samples of LD that were collected and stored between 2 and 10°C. The values were measured 4 times a day, at 3h intervals for 3 days. Stability was evaluated with a metrological criterion according to the intra-trial and inter-daily analytical variation and a biological criterion based on intra-individual biological variation.
StatisticsAll data were introduced in an Excel database and mean values and standard deviation (SD) were calculated. A data comparison was performed by Student t.
ResultsConcentrations obtainedThe results are shown in Table 1 and Fig. 1. The rest biochemical determinations (results not shown) matched the expected values of LD without P added.
The [P] obtained in the LD with citrate (n=10) was mean (SD) 0.38 (0.21) mg/dl. After further evaluation, it was found that the dilution was 1:200. It would have been needed 70ml of enema to obtain a [P]LD=1.5mg/dl.
Laboratory methodsThere were no statistically significant differences according to the analyser used. The stability study showed that the analysed samples remained stable during the 72h of study period.
Practical problems- (a)
In relation to the preparation of the LD: The AK200® monitors did not pass the initial tests if the enema is added when the monitor is being turned-on; it is necessary to reinitiate the monitor with an acid concentrate with no enema added and the enema should be added 10min later.
- (b)
Addition of the enema:
- •
In some cases, enema was added to containers that were not totally full, consequently higher [P]LD were obtained.
- •
The Casen® enema is marketed in a flexible plastic container with a cannula that cannot be completely emptied, so the actual volume administered may be less than that calculated.
- •
In one container of litre citrate acid (SelectBag Citrate®), with 50ml, a LD with [P]LD of 1.5mg/dl cannot be achieved with Casen® enema.
- (c)
No problems were reported during the HD session.
This manuscript presents an easy and practical method to calculate the volume of enema that needs to be added to the LD to obtain a target concentration of [P]LDobj.
Several publications show that adding P to the LD is a safe method to prevent the loss of P during the HD procedure.10,11 Its utility is evident and there are variety of clinical indications.9 We used a [P]LDobj of 1.5–2mg/dl if pre-dialysis serum P were maintained below 2.5mg/dl despite dietary intervention. There were several unanswered questions: how to add phosphate?; The calculation of how much enema should be added?, When?, Does it affect the monitor?, Can I measure the actual P concentration in the LD? All these questions were addressed in our study.
We decided to add P in the form of a Casen® enema because as compared with other commercial preparations the number of excipients is lower, it has a high P concentration. Casen® enema contains methyl parahydroxybenzoate that acts against moulds and yeasts; it is used in foods, cosmetics, and drugs and may produce allergic reactions. In our patients we have not seen adverse events, so we do not believe that it is a contraindication for its use. The Casen® enema does not contain endotoxins and cultures were negative.10 If the amount of phosphate in other preparations known, the calculation will be the same, but we have used only Casen® enema.
Our results show that the calculated volume is correct and easy to do. The difference between the P obtained and the calculated P is minimal; if the values were different there was an identifiable error that could have been anticipated. The experience with citrate illustrate that it is necessary to know the dilution of LD, and to be aware of any modification in the monitor.
Practice showed that with some monitors the enema cannot be added to the acid concentrate before the monitor is turned on. The enema adds sodium and other substances that, without modifying the electrolytic composition of the LD, may affect the initiation process of the AK200® monitor; although, we do not have an explanation for this. Waiting 10min before addition of the enema avoids the problem with the monitor, there is a minimal consumption of volume and does not alter the [P]LDobj. This operation may make nursing work just a little more difficult. The one-litre citrate containers do not have enough capacity to receive the adequate volume of enema.
Prior publications indicates that it is necessary to shake vigorously, even during 10–15min, to assure the dilution of the enema12; in our experience, such a vigorous shaking is not necessary.
Other were relate to the volume administered. First, adding the enema calculated in volumes of acid that were lower than anticipated resulted in higher [P]LD. This may occur if the volume of acid remaining in the bottles is not discarded. For example, adding 55ml of enema in 2l of acid will produce a LD with [P]LD of 2.6mg/dl instead of 1.5mg/dl. Second, it is necessary to avoid human errors by checking the volume administered, being careful of the commercial presentation and the need to use different volumes according to acid and the [P]LDobj. Finally, it the enema is added directly with the cannula, some volume may be left en the recipient producing underdosing.
We would like to emphasise that the aim of our work is not to recommend the administration of P in LD for all patients. In fact, in patients with serum P lower that 2.5mg/dl it is necessary to check dietary problems and P binders. The purpose of adding P to the dialysate is to avoid a negative P balance in patients with chronic hypophosphatemia. It remains to be seen whether it would be beneficial to produce P repletion or positive balance.
There is no information on what should be the P level after dialysis; in our opinion a post dialysis serum P ranging from 1 to 2mg/dl, may be appropriate taking into account the rebound of P. Although without symptomatology, a serum P less than 1mg/dl, P depletion may cause cell malfunction that is not easy to detect. In any case, we do not use LD concentrations greater than 2mg/dl, in patients with pre-dialysis serum P that are not greater than 2.5–3mg/dl unless the intake increases. Nevertheless the post-dialysis serum P concentration can be measured to prove that a satisfactory phosphatemia has been achieved at the end of the session. The post dialysis serum P using 1.5–2mg/dl of P in the dialysate have not exceeded concentrations of 2.5mg/dl.
Finally, the determination of [P]LD will assure that P being administered is adequate. This is an easy and reproducible method to demonstrate that our practice is correct. Other published works do not discuss this aspect. The fact that the sample of dialysate fluid is stable there is no need for centrifugation facilitates its measurement in non-hospital environments.
The main limitation of our data is that we have not determined the phosphatemias achieved, although this was not the aim of the work. Understanding the short/long term effect it may have opens up an interesting field of work. We also want to emphasise that the formula can only be used with the Casen® enema, since the denominator is determined by the content of P in the enema, but the calculation process would be the same using other products.
In summary, we believe that this work provides a practical view of how to deliver P in the LD, with a simple calculation, and presents the day to day problems that may appear and are not shown in any of the other works published. We believe that the number of determinations made is sufficient and shows that the calculation is correct, and offers measurement methods that can be used as quality control.
Conflicts of interestNone.
To the nursing staff of the Dialysis Unit and the technical staff of the laboratory of BrSalud del Hospital Universitario Infanta Leonor [BrSalud Princess Leonor University Hospital].
Please cite this article as: Albalate M, Ruiz-Alvarez MJ, de Sequera P, Perez-Garcia R, Arribas P, Corchete E, et al. Receta para prescribir fósforo durante hemodiálisis. Nefrologia. 2017;37:34–38.