This study was to explore the potential relationship between the fibrinogen-to-albumin ratio (FAR) and the presence and severity of coronary artery disease (CAD) in stage 3–5 predialysis chronic kidney disease (CKD) patients.
DesignThis study included 978 patients undergoing coronary angiography (CAG). CAD was defined as the presence of obstructive stenosis>50% of the lumen diameter in any of the four main coronary arteries. Gensini scores (GSs), left main coronary artery (LMCA) and three-vessel coronary artery disease (TVD) were used to elevate the severity of CAD.
ResultsThe adjusted odds ratios of CAD were 3.059 (95% CI: 1.859–5.032) and 2.670 (95% CI: 1.605–4.441) in the third and fourth quartiles of FAR compared with the first quartile, respectively. Among 759 patients diagnosed with CAD, multivariate logistic regression analysis showed that FAR (at the 0.01 level) was significantly positively associated with the presence of LMCA (adjusted OR=1.177, 95% CI 1.067–1.299, P=0.001) or TVD (adjusted OR=1.154, 95% CI 1.076–1.238, P<0.001), and a higher GS (adjusted OR=1.152, 95% CI 1.073–1.238, P<0.001).
ConclusionsFAR levels were independently associated with the presence and severity of CAD in stage 3–5 predialysis CKD patients.
Este estudio pretendía explorar la relación potencial entre la relación fibrinógeno/albúmina (FAR) y la presencia y la gravedad de la enfermedad arterial coronaria (EAC) en pacientes con enfermedad renal crónica (ERC) en estadio 3-5 en la etapa prediálisis.
DiseñoEste estudio incluyó a 978 pacientes tratados mediante angiografía coronaria. La EAC se definió como la presencia de estenosis obstructiva > 50% del diámetro de la luz de cualquiera de las 4 arterias coronarias principales. Se utilizaron las puntuaciones de Gensini (GS), la enfermedad de la arteria coronaria izquierda (EACI) y la EAC de 3 vasos (ETV) para evaluar la gravedad de la EAC.
ResultadosLos cocientes de posibilidades de EAC fueron 3,059 (IC del 95%: 1,859-5,032) y 2,670 (IC del 95%: 1,605-4,441) en el tercer y el cuarto cuartiles de la FAR en comparación con el primer cuartil, respectivamente. Entre los 759 pacientes diagnosticados de EAC, el análisis de regresión logística de múltiples variables mostró que la FAR (al nivel 0,01) presentaba una asociación positiva significativa con la presencia de EACI (OR ajustada = 1,177, IC del 95%: 1,067-1,299, p = 0,001) o ETV (OR ajustada=1,154, IC del 95%: 1,076-1,238, p < 0,001) y una puntuación GS mayor (OR ajustada = 1,152, IC del 95%: 1,073-1,238, p < 0,001).
ConclusionesLos niveles de FAR se asociaron de manera independiente con la presencia y la gravedad de EAC en los pacientes con ERC en estadio 3-5 en la etapa prediálisis.
Chronic kidney disease (CKD) has recently emerged as a major worldwide public health concern.1 Coronary artery disease (CAD) remains the most common cause of morbidity and mortality among CKD patients.2 Several studies have identified that the incidence of CAD in CKD patients was significantly higher than in patients without CKD, with inflammation serving as one of its important pathological mechanisms.3,4 Identifying valuable markers of inflammation is important in the prevention and early treatment of CAD in CKD patients.
The fibrinogen-to-albumin ratio (FAR), the combination with fibrinogen and albumin, is a probable inflammation marker to predict cardiovascular diseases. Several studies have shown that FAR had an association with the severity of coronary artery disease (CAD).5,6 Among patients undergoing primary percutaneous coronary intervention (PCI), FAR was associated with the in-stent restenosis7 and the major adverse cardiovascular events (MACEs).8 Moreover, recent research has found the association between FAR and the prognostic value of several types of human cancers,9,10 the activity of Ankylosing Spondylitis11 and the severe illness due to corona virus disease 19.12
Patients with CKD have higher levels of fibrinogen and elevated clot strength.13 However, the serum albumin level is negatively associated with renal function in CKD patients.14 Clinical data on the impact of FAR levels on the presence and severity of CAD among CKD patients are scarce. Muntner et al. found that higher levels of fibrinogen and lower levels of serum albumin were positively associated with coronary heart disease among CKD patients, while the FAR levels was not studied.15 This cross sectional-study was to explore the relationship between FAR levels and CAD in stage 3–5 predialysis CKD patients.
MethodsStudy populationThe estimated glomerular filtration rate (eGFR) was estimated according to the Kidney Disease-Epidemiology Collaboration (CKD-EPI) formula16 and those patients whose eGFR were less than 60mL/min/1.73m2 were defined as CKD. Of all the 6749 patients who received coronary angiography (CAG) due to angina-like chest pain, the consecutive data of 521 CKD patients at The People's Hospital of Suzhou New District (date range: January 2017 and November 2019) and 457 CKD patients at the Zhong Da Hospital Affiliated to Southeast University (date range: May 2016 and December 2016) who did not undergo dialysis were analyzed. The exclusion criteria comprised of hepatic failure, evidence of an active infection or systemic inflammatory disease, valvular disease, cardiomyopathy, myocarditis, thyroid dysfunction, cancer, acute renal insufficiency, a history of kidney transplant or previous CAD. Of all the 978 CKD patients, 70 patients had acute coronary syndromes.
Medical histories were obtained by reviewing the patients’ electronic medical records and included the age, sex, body mass index (BMI), history of smoking, alcohol-intake, hypertension, and diabetes mellitus. Hypertension was diagnosed as a repeated blood pressure measurement>140/90mmHg or based on antihypertensive drug medication. Diabetes mellitus was diagnosed as repeated fasting serum glucose levels>7.0mmol/L, a random plasma glucose level>11.1mmol/L, or on hypoglycemic medication.
Laboratory measurementsBefore CAG, patients’ fasting blood samples were obtained from the antecubital vein after fasting for 8h. The complete blood count was analyzed by the automated hematology analysis system (Beckman LH750, Beckman Coulter, Brea, CA, USA; Sysmex DI-60, Sysmex Corporation, Kobe, Japan). Laboratory indices including hemoglobin, uric acid, triglyceride (TC), total cholesterol (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein-cholesterol (LDL-C), lipoprotein (a) [Lp(a)], serum creatinine (Scr), prealbumin, and albumin levels were tested with a biochemistry analyzer (Beckman AU680, Beckman Coulter; Roche Cobas 8000, Roche, Basel, Switzerland). Plasma fibrinogen levels were measured using the Clauss’ method (Beckman ACL-TOP700, Beckman Coulter; C3510, Mairui, Beijing, China).
Coronary angiographyThe CAG was performed using an X-ray system according to the standard Judkins technique (Philips UNIQ Clarity FD10, Philips, Amsterdam, Netherlands; Siemens ARTIS Icono Biplane, Munich, Germany) and all patients had signed the informed consent form. At least two different plane images were obtained for the main coronary artery, including the left main coronary artery (LM), the left anterior descending coronary artery (LAD), the left circumflex coronary artery (LCX), and the right coronary artery (RCA). CAD was defined as the presence of obstructive stenosis>50% of the lumen diameter in any of aforesaid four main coronary arteries. Patients with a stenosis≥50% in diameter in the LM was diagnosed as LM coronary artery stenosis (LMCA). Simultaneous stenosis (≥50%) of the LAD, LCX, and RCA, with or without LM involvement, was diagnosed as three-vessel coronary artery disease (TVD). LMCA and TVD were defined as severe coronary artery stenosis (CAS).
The Gensini Score (GS) was calculated for patients diagnosed with CAD (n=759) to evaluate the severity of CAS.17 Each coronary artery had a coefficient that corresponded to the lesion's position, such as 5 for LM; 2.5 for proximal LAD and proximal LCX; 1.5 for the midportion LAD; 1 for the RCA, the distal segment of the LAD, the mid-distal region of the LCX, the posterolateral artery, and the obtuse marginal artery; and 0.5 for other segments. These coefficients were multiplied by 1, 2, 4, 8, 16, and 32, and were associated with 0–25%, 26–50%, 51–75%, 76–90%, 91–99%, and 100% stenosis, respectively. The total score for each patient was calculated. The lower GS group (n=378) was defined as a GS between 1 and 30, and the higher GS group (n=381) was defined as a GS>30 according to the median of the subjects diagnosed with CAD in the study.
Statistical analysisThe statistical analyses were conducted with the SPSS 25.0 software package (IBM Corporation, Armonk, NY, USA). Normally distributed variables were expressed as means±standard deviations and compared using Student's t-test. Non-normal variables were presented as medians with interquartile ranges, and the Mann–Whitney U-test was used. The categorical variables were presented as numbers and percentages and were compared using Fisher's exact tests or chi-squared tests. The Kruskal–Wallis rank test and chi-squared test were used to compare continuous variables and categorical variables related to FAR quartiles, as appropriate. Correlation analysis between FAR and GS was performed using Spearman's correlation coefficient. Multivariate logistic regression analysis was used to determine the relationship between FAR levels and the presence and severity of CAD. A P-value<0.05 was considered as statistically significant.
ResultsBaseline clinical characteristicsA total of 978 CKD patients who had undergone CAG were divided into one of two groups: the CAD group (n=759) and the non-CAD group (n=219). As shown in Table 1, the mean age of all patients was 73.6 years, and the mean eGFR value was 50.4mL/min/1.73m2. Patients with CAD were older than patients without CAD (P<0.001), and they were more likely to be males, smokers, or had a history of hypertension and diabetes mellitus (P<0.001). Moreover, the FAR levels were significantly higher in the CAD group than in the non-CAD group (P<0.001). In contrast, the levels of eGFR in the CAD group were significantly lower than those in the non-CAD group (P<0.001). Serum levels of uric acid, lipoprotein (a) (Lp[a]) and fibrinogen were higher in patients with CAD than in non-CAD patients (P<0.05), while patients with CAD had lower HDL-C, prealbumin, and albumin levels (P<0.05). There were no significant differences of BMI, systolic blood pressure, diastolic blood pressure, hemoglobin, TG, TC, LDL-C, and the presence of alcohol-intake between the two groups (P>0.05).
Baseline clinical characteristics.
| Variables | Total patients (n=978) | Non-CAD (n=219) | CAD (n=759) | P |
|---|---|---|---|---|
| Age (years) | 73.6±8.6 | 71.0±8.8 | 74.4±8.4 | <0.001 |
| Male, n (%) | 479 (49.0) | 74 (33.8) | 405 (53.4) | <0.001 |
| Smoking, n (%) | 223 (22.8) | 21 (9.6) | 202 (26.6) | <0.001 |
| Alcohol-intake, n (%) | 69 (7.1) | 9 (4.1) | 60 (7.9) | 0.053 |
| Hypertension, n (%) | 758 (77.5) | 147 (67.1) | 611 (80.5) | <0.001 |
| Systolic blood pressure (mmHg) | 137.2±22.3 | 136.4±19.6 | 137.4±23.0 | 0.559 |
| Diastolic blood pressure (mmHg) | 78.5±31.9 | 78.9±11.8 | 78.4±35.7 | 0.834 |
| Diabetes mellitus, n (%) | 297 (30.4) | 34 (15.5) | 263(34.7) | <0.001 |
| BMI (kg/m2) | 25.5±3.8 | 25.6±4.2 | 25.5±3.7 | 0.672 |
| Hemoglobin (g/L) | 129.1±18.1 | 130.1±16.8 | 128.8±18.4 | 0.345 |
| eGFR (mL/min/1.73m2) | 50.4 (42.6–56.0) | 52.9 (46.1–57.3) | 49.7 (41.5–55.5) | <0.001 |
| Uric acid (mg/dL) | 6.7±2.0 | 6.4±2.2 | 6.8±1.9 | 0.033 |
| TG (mmol/L) | 1.7±1.1 | 1.6±1.1 | 1.7±1.1 | 0.588 |
| TC (mmol/L) | 4.2±1.1 | 4.3±1.0 | 4.2±1.2 | 0.077 |
| HDL-C (mmol/L) | 1.2±0.3 | 1.2±0.3 | 1.1±0.3 | 0.002 |
| LDL-C (mmol/L) | 2.6±0.9 | 2.7±0.8 | 2.6±0.9 | 0.143 |
| Lp(a) (mg/L) | 258.5 (154.8–424.0) | 197.0 (132.0–350.0) | 267.0 (167.0–457.0) | <0.001 |
| Prealbumin (mg/L) | 167±63 | 176±58 | 165±64 | 0.016 |
| Albumin (g/L) | 40.0±4.1 | 40.8±3.6 | 39.7±4.2 | 0.001 |
| Fibrinogen (g/L) | 3.9±0.8 | 3.6±0.8 | 4.0±0.8 | <0.001 |
| FAR | 0.097 (0.082–0.112) | 0.088 (0.076–0.101) | 0.100 (0.085–0.114) | <0.001 |
The data are expressed as the n (%), the mean±the standard deviation (SD), or the median (25–75th percentile). CAD, coronary artery disease; BMI, body mass index; eGFR, estimated glomerular filtration rate; TG, triglycerides; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; Lp(a), lipoprotein (a); FAR, fibrinogen-to-albumin ratio.
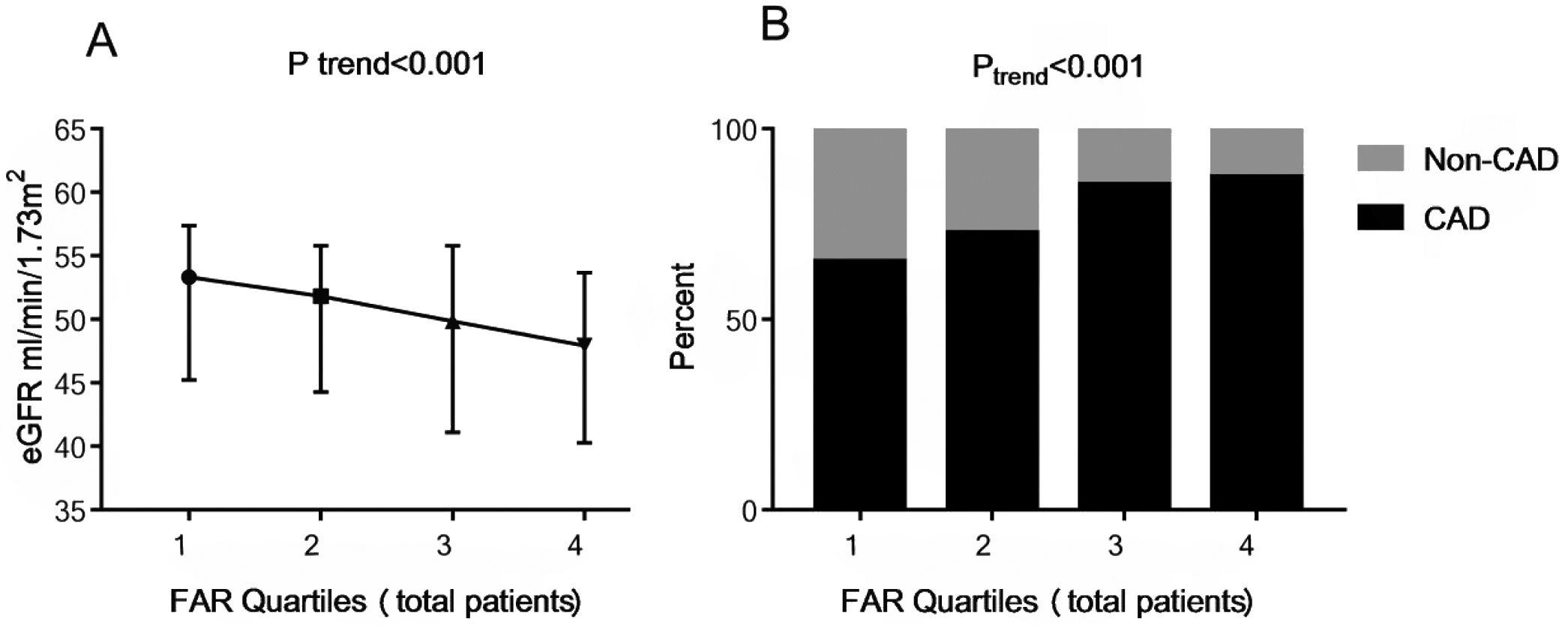
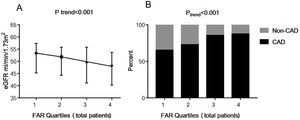
In the correlation analysis, FAR was negatively associated with eGFR (r=−0.195, P<0.001). Then the study subjects were further grouped based on FAR quartile distributions (Quartile 1: 0.045–0.082; Quartile 2: 0.083–0.096; Quartile 3: 0.097–0.111; Quartile 4: 0.112–0.212). As shown in Fig. 1A, patients with lower FAR levels had higher levels of eGFR (53.3 [interquartile range, 45.3–57.4] vs. 51.8 [44.3–55.8] vs. 49.8 [41.1–55.8] vs. 47.9 [40.3–53.7]mL/min/1.73m2, Ptrend<0.001).
The eGFR and the presence of CAD among FAR quartiles. Quartiles for total patients (n=978): Quartile 1: 0.045–0.082; Quartile 2: 0.083–0.096; Quartile 3: 0.097–0.111; Quartile 4: 0.112–0.212. Data were reported as the percent or median (interquartile range). eGFR, estimated glomerular filtration rate; FAR, fibrinogen-to-albumin ratio; CAD, coronary artery disease.
As shown in Fig. 1B, the percent of CAD was significantly higher in the groups with higher FAR levels, with 65.2%, 72.7%, 85.3% and 87.3% in the FAR quartile 1–4, respectively (Ptrend<0.001). In the multivariate logistic regression analysis shown in Table 2, FAR (at the 0.01 level, adjusted OR=1.191, 95% CI 1.100–1.290, P<0.001) and higher FAR quartiles (quartile 3 vs. quartile 1 [adjusted OR=3.059, 95% CI 1.859–5.032, P<0.001]; quartile 4 vs. quartile 1 [adjusted OR=2.670, 95% CI 1.605–4.441, P<0.001]) were significantly positively associated with the prevalence of CAD after adjusting for related factors, such as age, sex, BMI, smoking, alcohol-intake, hypertension, systolic blood pressure, diastolic blood pressure, diabetes mellitus, eGFR, uric acid, TG, TC, HDL-C, LDL-C, hemoglobin, prealbumin, and Lp(a). Moreover, age, male, and a history of smoking, hypertension, and diabetes mellitus had a positive association with the presence of CAD (P<0.05).
Multivariate logistic regression analysis to assess the correlation between FAR quartiles and the presence of CAD, LMCA, TVD, and higher GS.
| Outcome | Adjusted OR (95% CI) | P |
|---|---|---|
| Total study patients (n=978) | ||
| CAD (759, 77.6%) | ||
| FAR*100 | 1.191 (1.100–1.290) | <0.001 |
| FAR quartiles | ||
| Quartile 1 | 1.0 (ref) | |
| Quartile 2 | 1.302 (0.851–1.991) | 0.233 |
| Quartile 3 | 3.059 (1.859–5.032) | <0.001 |
| Quartile 4 | 2.670 (1.605–4.441) | <0.001 |
| Patients with diagnosed CAD (n=759) | ||
| LMCA (64, 8.4%) | ||
| FAR*100 | 1.177 (1.067–1.299) | 0.001 |
| FAR quartiles | ||
| Quartile 1 | 1.0 (ref) | |
| Quartile 2 | 1.827 (0.732–4.560) | 0.196 |
| Quartile 3 | 1.661 (0.666–4.141) | 0.277 |
| Quartile 4 | 3.274 (1.406–7.627) | 0.006 |
| TVD (326, 43.0%) | ||
| FAR*100 | 1.154 (1.076–1.238) | <0.001 |
| FAR quartiles | ||
| Quartile 1 | 1.0 (ref) | |
| Quartile 2 | 1.389 (0.867–2.226) | 0.172 |
| Quartile 3 | 1.701 (1.072–2.699) | 0.024 |
| Quartile 4 | 2.777 (1.723–4.475) | <0.001 |
| Higher GS (381, 50.2%) | ||
| FAR*100 | 1.152 (1.073–1.238) | <0.001 |
| FAR quartiles | ||
| Quartile 1 | 1.0 (ref) | |
| Quartile 2 | 1.389 (0.877–2.199) | 0.161 |
| Quartile 3 | 2.141 (1.353–3.385) | 0.001 |
| Quartile 4 | 2.260 (1.399–3.652) | 0.001 |
Adjusted for age, sex, BMI, smoking, alcohol-intake, hypertension, systolic blood pressure, diastolic blood pressure, diabetes mellitus, eGFR, uric acid, TG, TC, HDL-C, LDL-C, hemoglobin, prealbumin, and Lp(a). Regression model: forward stepwise.
95% CI, 95% confidence interval; OR, odds ratio; FAR, fibrinogen-to-albumin ratio; quartiles for all patients: Quartile 1: 0.045–0.082; Quartile 2: 0.083–0.096; Quartile 3: 0.097–0.111; Quartile 4: 0.112–0.212; FAR*100 was defined as the concentration ratio of fibrinogen (g/L) to albumin (g/l) multiplied by 100. Quartiles for CAD patients: Quartile 1: 0.045–0.085; Quartile 2: 0.086–0.099; Quartile 3: 0.100–0.114; Quartile 4: 0.115–0.212; higher GS: GS>30. CAD, coronary artery disease; BMI, body mass index; eGFR, estimated glomerular filtration rate; TG, triglycerides; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; Lp(a), lipoprotein (a); LMCA, left main coronary artery stenosis; TVD, three-vessel coronary artery disease; GS, Gensini score.
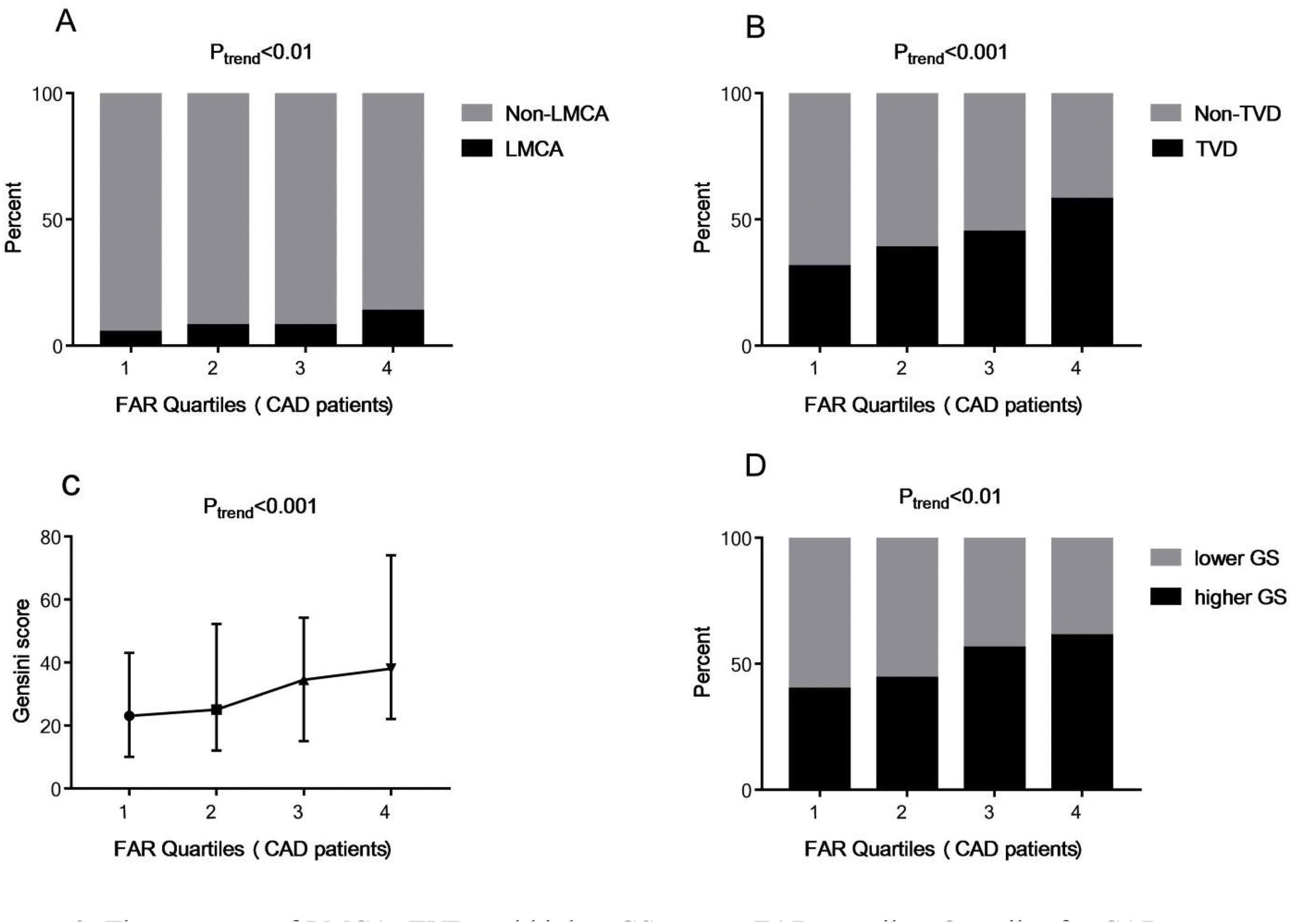
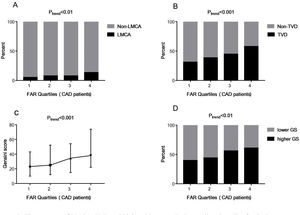
To explore the association between FAR levels and the severity of CAD, 759 patients diagnosed with CAD were grouped according to FAR quartile distributions (Quartile 1: 0.045–0.085; Quartile 2: 0.086–0.099; Quartile 3: 0.100–0.114; Quartile 4: 0.115–0.212). As shown in Fig. 2A and B, those subjects with higher FAR levels showed higher percent of LMCA (5.1% vs. 7.7% vs. 7.7% vs. 13.4% in the FAR quartile 1–4 groups respectively, Ptrend<0.01) and TVD (31.1% vs. 38.5% vs. 44.8% vs. 57.8% in the FAR quartile 1–4 groups respectively, Ptrend<0.001). Moreover, FAR was positively associated with GS (r=0.232, P<0.001). Fig. 2C shows that the GS values were increased with the increase of FAR quartile distributions (23.00 [interquartile range, 10.00–43.00] vs. 25.00 [12.00–52.25] vs. 34.5 [15.0–54.25] vs. 38.00 [22.00–74.00], Ptrend<0.001).
The presence of LMCA, TVD and higher GS among FAR quartiles. Quartiles for CAD patients (n=759): Quartile 1: 0.045–0.085; Quartile 2: 0.086–0.099; Quartile 3: 0.100–0.114; Quartile 4: 0.115–0.212. Data were reported as the percent or median (interquartile range). Higher GS: GS>30; lower GS: GS 1–30; FAR: fibrinogen-to-albumin ratio; CAD: coronary artery disease; LMCA: left main coronary artery stenosis; TVD: three-vessel coronary artery disease; GS: Gensini score.
To examine the role of FAR in the severity of CAD, multivariate regression analysis was performed in our study. As shown in Table 2, after adjusting for abovementioned confounders, FAR (at the 0.01 level) was significantly positively associated with the prevalence of LMCA (adjusted OR=1.177, 95% CI 1.067–1.299, P=0.001) and TVD (adjusted OR=1.154, 95% CI 1.076–1.238, P<0.001). Likewise, FAR quartiles were independently associated with the prevalence of LMCA (quartile 4 vs. quartile 1 [adjusted OR=3.274, 95% CI 1.406–7.627, P=0.006]) and TVD (quartile 3 vs. quartile 1 [adjusted OR=1.701, 95% CI 1.072–2.699, P=0.024]; quartile 4 vs. quartile 1 [adjusted OR=2.777, 95% CI 1.723–4.475, P<0.001]).
Moreover, we further divided the subjects into two groups according to the median of GS: the lower GS group (GS 1–30, n=378) and the higher GS group (GS>30, n=381). As shown in Fig. 2D, the percent of higher GS patients was significantly higher in the groups with higher FAR levels, which were 39.8%, 44.0%, 56.2% and 61.0% in the FAR quartile 1–4 groups respectively (Ptrend<0.001). In the multivariate logistic regression analysis, FAR (at the 0.01 level, adjusted OR=1.152, 95% CI 1.073–1.238, P<0.001) and higher FAR quartiles (quartile 3 vs. quartile 1 [adjusted OR=2.141, 95% CI 1.353–3.385, P=0.001]; quartile 4 vs. quartile 1 [adjusted OR=2.260, 95% CI 1.399–3.652, P=0.001]) were also independently associated with the prevalence of higher GS.
DiscussionIn the study, we found that there was an independent association between FAR and the prevalence of CAD. Moreover, FAR was significantly associated with the severity of angiography-proven CAD presented as LMCA, TVD, and a higher GS.
Previous studies showed that eGFR levels were negatively associated with fibrinogen and positively related to serum albumin levels.18 Furthermore, both higher fibrinogen and lower serum albumin levels were associated with atherosclerotic vascular disease and death in CKD patients.19 Therefore, FAR, derived from the ratio of fibrinogen to albumin, may better reflect the inflammatory status in CKD patients. In our study, we found a significant negative correlation between FAR and renal function in stage 3–5 predialysis CKD patients before CAG, which was consistent with the previous studies.
Previous researchers found that increased FAR levels are significantly associated with cardiovascular disease. Xiao et al. analyzed the data of 278 ST-segment elevation myocardial infarction (STEMI) patients and revealed that FAR was significantly related to Synergy between PCI and the Taxus and Cardiac Surgery Study (SYNTAX) score in predicting the severity of CAD.20 Celebi et al. found that CAD patients with higher SYNTAX scores had higher FAR levels.5 However, the role of FAR in CKD patients has not been previously investigated. In the present study, we found that the group with higher FAR levels had higher prevalence of CAD among CKD patients after adjusting for confounding factors. We found that higher FAR levels were positively associated with the higher GS, which is similar to the previous results obtained for fibrinogen.21 Besides the GS, we also included the location and number of coronary artery lesions to evaluate the severity of CAD, which was not referred to in previous studies. In this study, we found that the prevalence of LMCA and TVD were significantly increased across quartile distributions of FAR levels. In the multivariate logistic regression analysis, higher FAR quartiles were still significantly associated with the prevalence of LMCA and TVD.
While the normal FAR level in CKD patients remains unknown, a recent study based on 1352 non-STEMI patients found the cut off value of FAR to be 0.091 when predicting MACEs.22 In the present study, we grouped according to FAR quartile distributions to explore its relationship with CAD and there was no statistically significant correlation between FAR quartile 2 and quartile 1 in our study. The specific value and cut-off value of FAR need to be authenticated through basic experiments and multi-center clinical cohort studies.
The possible pathological mechanism of FAR in CAD is its pro-inflammatory and pro-coagulant roles. CKD patients were more likely to promote the formation of atherosclerotic plaque because of the abnormal FAR levels. The pathological processes of coronary atherosclerosis include the accumulation of foam cells, the formation of fatty streaks and fibrous plaques, the rupture of acute plaques, and thrombosis.23 Substantial evidence showed an association between chronic inflammation and CAD, as a large number of inflammation mediators are released and play a critical role throughout these aforementioned steps in atherogenesis steps.24 To our knowledge, fibrinogen, the precursor of fibrin, participates in multiple coagulation processes, such as platelet aggregation, fibrin formation, coagulation activation, and fibrinolysis.25 Fibrinogen is also a cofactor of platelet aggregation and an acute-phase protein, which plays an important role in the CAD due to lipid peroxidation.26,27 Serum albumin has physiological anti-inflammatory, anti-oxidation, anti-coagulation, and anti-platelet aggregation properties, which play a negative role in the development of CAD.28,29
To our knowledge, this was the first study based on FAR and CAD among a large sample size of stage 3–5 predialysis CKD patients. However, several limitations should be noted. First, the major limitation of this study was its retrospective nature, therefore, we could not draw a causal conclusion between FAR and CAD. Second, it should be mentioned that this study measured only the initial value of FAR, while its changes over time may provide additional prognostic value. Third, there was no statistical analysis of prognosis and endpoint events, such as in-stent restenosis, MACEs, cardiogenic death, and all-cause death. Therefore, future prospective studies that investigate additional influencing factors are needed to provide additional prognostic value.
ConclusionIn the present cross-sectional study conducted among patients with stage 3–5 predialysis CKD, there was an independent association between FAR and the prevalence of angiography-proven CAD. Moreover, FAR was significantly associated with the severity of CAD presented as LMCA, TVD, and a higher GS.
FundingThe Key Project of Scientific Innovation Fund of The People's Hospital of Suzhou New District, Suzhou, China (grant no. SGY2020A03).
Conflict of interestThe authors declare that they have no conflicts of interest and no relevant financial disclosures. The results presented in this paper have not been published previously in whole or part, except in abstract form.
We are grateful to the staff of the department of nephrology at The People's Hospital of Suzhou New District and Zhongda Hostipal. We are also grateful for every co-author making contributions to the article. This work was supported the Key Project of Scientific Innovation Fund of The People's Hospital of Suzhou New District, Suzhou, China [grant number SGY2020A03].