Maintenance intravenous fluids are frequently used in hospitalised pediatric patients. The aim of the study was to describe the adverse effects of isotonic fluid therapy in hospitalised patients, and its prevalence based on the rate of infusion.
Materials and methodsA prospective clinical observational study was designed. We included hospitalised patients between 3 months-old and 15-years-old were included with 0,9% isotonic solutions with 5% glucose within the first 24 h of administration. They were divided into two groups, depending on the quantity of liquid they received (restricted <100% vs 100% maintenance needs). Clinical data and laboratory findings were recorded in two different times (T0 when they were admitted to hospital and T1 within the first 24 h of administration).
ResultsThe study included 84 patients, 33 received <100% maintenance needs and 51 patients received around 100%. The main adverse effects notified in the first 24 h of administration were hyperchloremia >110 mEq/L (16.6%) and oedema (19%). Oedema was more frequent in patients with lower age (p < 0,01). The hyperchloremia at 24 h of intravenous fluids was an independent risk factor of developing oedema (OR 1,73 (1,0–3,8), p = 0,06).
ConclusionThe use of isotonic fluids is not free from adverse effects, probably related to the rate of infusion and more likely to appear in infants. It`s necessary more studies that review the correct estimation of intravenous fluid needs in hospitalized children.
La fluidoterapia intravenosa es un tratamiento ampliamente utilizado en pacientes pediátricos hospitalizados. El objetivo del estudio fue analizar las complicaciones asociadas al uso de sueros isotónicos de mantenimiento en pacientes hospitalizados y comparar la frecuencia de aparición de estas complicaciones con distintos ritmos de administración.
Materiales y métodosSe realizó un estudio observacional y prospectivo, en el que se incluyeron pacientes hospitalizados de entre 3 meses y 15 años de edad que recibieron tratamiento con fluidoterapia isotónica 0,9% con glucosa al 5% durante las primeras 24 horas de ingreso. Se dividieron en dos cohortes según el ritmo de fluidoterapia: restringido <100% vs no restringido cercano al 100% NNBB, calculadas según la regla de Holliday y Segar. Se recogieron variables clínicas y analíticas en dos tiempos de estudio (T0 al ingreso y T1 a las 24 horas de la fluidoterapia). Se realizó un estudio uni y multivariante para identificar factores de riesgo de complicaciones.
ResultadosSe incluyeron 84 pacientes, de los cuales 33 recibieron fluidoterapia restringida y 51 pacientes con 100% NNBB. Las principales complicaciones desarrolladas en las primeras 24 horas fueron hipercloremia >110 mEq/L (16,6%) y edemas (19%). La aparición de edemas fue más frecuente en pacientes de menor edad (p < 0,01) y la hipercloremia se asoció con el desarrollo de edemas (OR 1,73 (1,0–3,8), p = 0,06.
ConclusionesLa administración de sueros isotónicos no está exenta de complicaciones, probablemente relacionadas con el ritmo de administración y más frecuentes en lactantes. Son necesarios estudios que revisen las necesidades de líquidos en niños hospitalizados.
Intravenous fluid therapy is a frequent treatment in pediatric hospitalization wards, both for intravenous rehydration and for maintaining hydroelectrolyte needs.1,2 It must be used with caution and be considered as a medication, in which both the composition of the serum (isotonic or hypotonic) and the volume or rhythm in which it is administered must be adjusted to the characteristics of each individual patient.3 Most of the studies focus on recommending an adequate tonicity for the fluids, but there are very few articles that evaluate the adequate rhythm of administration of maintenance replacement fluids.
The clinical practice guidelines of the American Academy of Pediatrics of 2018 recommend the use of isotonic fluids for maintenance fluid therapy with the appropriate addition of potassium and glucose in general for patients between 28 days and 18 years of age, thus changing the previous paradigm of the use of hypotonic fluids in pediatrics.2 The main reason for this change was to avoid the complications associated with the use of hypotonic fluid, mainly iatrogenic hyponatremia, with associated risk of cerebral edema with the associated morbidity and mortality.2,4 However, there is limited amount of publications on the safety of the widespread use of isotonic fluids in pediatric wards, in stable non-post-surgical patients.5–12
The aim of this study was to analyze the complications associated with the use of maintenance isotonic fluids in patients admitted to pediatric hospitalization wards and to compare whether or not the complications were related to the rate of administration.
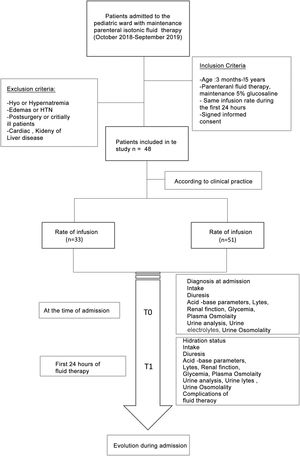
Materials and methodsIt was designed an observational and prospective study between October 2018 and September 2019.The study included patients admitted to the Pediatric Hospitalization ward of a tertiary hospital with maintenance intravenous fluid therapy with isotonic saline solution (0.9%) with 5% glucose (glucosaline 5%). This fluid contains 154 mEq/L sodium chloride and 50 g/L glucose with a calculated osmolarity of 586 mOsm/L.
The patients were divided into two cohorts based on the rate of fluid therapy: a first cohort with patients with restricted rate and a second cohort with an unrestricted rate close to 100% of their BFN calculated according to the Holliday and Segar formula.13 The decision of the rate of fluid therapy was made according to the usual clinical practice of the doctor who attended the patient at the time of admission.
The inclusion criteria were: age between three months and 15 years, treatment with 5% glucose saline solution without changes in the rate of administration during the first 24 h, and signed an informed consent for anonymous data collection.
Patients excluded were those who presented, before IV fluid therapy, dehydration secondary to acute gastroenteritis, edema, hypertension, alterations in serum sodium concentration in the analysis on admission, patients with underlying pathologies that made fluid management difficult (cardiac, renal, hepatic, oncological and adrenal insufficiency) and patients in critical condition or post-surgery. Patients younger than three months were also excluded since in these patients some authors recommend the use of hypotonic fluids.11,12
There were recorded the diagnosis on admission and the intake shown by the patient before and during the time that he received fluid therapy.
Two study times were considered: T0 at the time of admission (prior to the start of fluid therapy) and T1 24 h after IV fluid therapy.
There were documented the clinical variables (diuresis, intake, hydration, edema, weight) and analytical variables at both times of the study: venous blood gases (pH, bicarbonate), renal function, ions, plasma osmolarity, urinalysis (osmolarity, urine electrolytes). The patient's renal function was assessed according to the glomerular filtration rate estimated with the Schwartz formula adjusted for the height14 and renal damage was considered when the patient had a creatinine greater than 1.5 times the normal value for his age, according to the classification of pRIFFLE.15 Complications presented during the first 24 h of fluid therapy were collected, as well as the evolution of the patient until discharge (Fig. 1).
Statistical analysis was performed using the SPSS statistical program (IBM SPSS Statistics, Armonk, NY) version 25.0. Quantitative variables were expressed as median and interquartile range. Qualitative variables were expressed as percentages. The Fisher test was used to compare the qualitative variables and the Mann–Whitney U test and the Kruskall Wallis test were used for the quantitative variables. To assess the factors that could be associated to the presence of complications (edema), several probabilistic logistic regression models were built including all the variables close to statistical significance (p < 0.2) and clinically relevant ones. The results were expressed in the logistic regression model as OR and CI 95%. The Akaike indices (AIC) and the Bayesian information criteria (BIC) were used as selection and comparison criteria between the logistic regression models. The model chosen was the one with the lowest value of AIC and BIC.
To assess the presence of hyperchloremia, a linear regression study was performed whose dependent variable was chloremia on T1 and the independent variables were age, drip rate, glomerular filtration rate, and blood glucose at the start of fluid therapy. The results were expressed as t coefficients, β and p value. Collinearity was ruled out in all models. A p < 0.05 was considered statistically significant. The study was approved by the Clinical Research Ethics Committee of Hospital Gregorio Marañón (Code 266/18) and all patients or their families signed the informed consent.
ResultsGlobal sample analysisDescriptive analysis of the sampleDuring the study there were 84 patients included. The median age was 4.5 (1–13) years and 56% were male. The mean length of stay was 4 (2.2−6.5) days. Twenty-three percent of the sample had a personal history of interest, mostly neurological history (infantile cerebral palsy, psychomotor retardation, etc.). Regarding the clinical diagnosis on admission, 31% were patients had gastro-intestinal pathology who required a total intake restriction (abdominal pain without digestive losses), 31% infectious pathology and 18% respiratory pathology that prevented oral intake.
Analysis according to the rhythm of fluid therapyOf the 84 enrolled patients, 39.3% received fluid therapy restricted to less than 100% of BFN and the rest (60.7%) had rhythms close to 100% of their BFN. Patients with <100% BFN had a median rhythm of 75% (50–80%) of BFN and the unrestricted group had a median 100% (90–100%) of BFN. The distribution of patients according to rhythm is shown in Appendix B graph A of the supplementary material.
The clinical and analytical variables of the patients were analyzed according to the rhythm of fluid therapy (Table 1). Both groups were comparable at the time of fluid therapy indication (T0) in terms of age, personal history, diagnosis, renal function, and laboratory results on admission.
Bivariate comparative study of clinical and analytical variables of the patients according to the rhythm of intravenous fluid therapy.
| 100%-BFN rhythm (n = 51) | Restricted rhythm <100% BFN (n = 33) | p | |
|---|---|---|---|
| Age (years) | 3 (0.9−7.5) | 3 (0.5−13) | 0.85 |
| Average stay (days) | 4 (2.5−6.5) | 3 (2−5) | 0.17 |
| pH T0 | 7.36 (7.30−7.40) | 7.36 (7.33−7.40) | 0.39 |
| HCO3 T0 (mmol/L) | 22 (20.5−24.5) | 23 (20−24) | 0.94 |
| Na T0 (mmol/L) | 136 (134−138) | 137 (135−139) | 0.52 |
| K T0 (mmol/L) | 4.5 (3.9−5.35) | 4.2 (3.8−4.6) | 0.10 |
| Cl T0 (mEq/L) | 102 (100−105) | 102 (100−103) | 0.57 |
| Blood glucose T0 (mg/dL) | 96 (85−118) | 96 (88−103) | 0.86 |
| Urea T0 (mg/dL) | 23 (17−31) | 21 (15−32) | 0.65 |
| Creatinine T0 (mg/dL) | 0.36 (0.28−0.50) | 0.37 (0.25−0.62) | 0.52 |
| eGFR at T0 (mL/min/1.73 m2) | 97.12 (24.5−110.1) | 100.6 (100.6−114) | 0.52 |
| pH T1 | 7.38 (7.33−7.41) | 7.39 (7.35−7.40) | 0.56 |
| HCO3 T1(mmol/L) | 24 (22−25) | 22 (21−24) | 0.11 |
| Na T1 (mmol/L) | 139 (138−141) | 138 (136−140) | 0.12 |
| K T1 (mmol/L) | 4.2 (3.8−4.6) | 4.2 (3.9−4.5) | 0.89 |
| Cl T1 (mmol/L) | 107 (106−110) | 105 (103−107) | <0.01a |
| T1 blood glucose (mg/dL) | 98 (90−108) | 101 (90−114) | 0.39 |
| Urea T1 (mg/dL) | 13 (10−19) | 14 (7−21) | 0.79 |
| T1 creatinine (mg/dL) | 0.27 (0.21−0.45) | 0.25 (0.20−0.50) | 0.84 |
| eGFR at T1 (mL/min/1.73 m2) | 125 (108.6−156) | 128 (98−148) | 0.55 |
| Male gender (%) | 55% | 58% | 0.82 |
| Diagnosis on admission | |||
| Respiratory | 18% | 18% | |
| G.I. | 37% | 21% | 0.15 |
| Infectious | 24% | 42% | |
| Others | 21% | 19% | |
| -underlying pathologies (%) | 20% | 27% | 0.43 |
| Edema (%) | 20% | 18% | 0.55 |
BFN: basal fluid needs calculated according to Holliday-Segars.
The results are expressed in medians with interquantile range (p25–p75).
eGFR: estimated glomerular filtration rate using the 2009 updated Schwartz equation.
At T1, after the first 24 h of fluid therapy, the median plasma chloride concentrations was significant higher in patients with unrestricted fluids (100% NNBB) (107 (106−110) mmol/L) as compared with the group of restricted infusion rates (105 (103−107) mmol/L, (p < 0.01). (Table 1).
ComplicationsThe main complications of the administration of isotonic fluid therapy 24 h after admission were edema in 19% of patients and hyperchloremia in 16.6%. None developed hypernatremia >145 mmol/L and only 2.3% had mild hyponatremia <135 mmol/L. Hyperglycemia was present in 40% and plasma hyperosmolarity (>290 mosm/L) occured in 38% of patients.
A 16.6% of the patients presented hyperchloremia >110 mEq/L (range 111–119 mEq/L). 17.8% had acidosis (pH < 7.35) with no correlation with a decrease in bicarbonate. In T1 there was a reduction in anion GAP (p = 0.04) probably related to the hyperchloremia (p < 0.01). (Appendix B table A of the supplementary material).
The patients who developed edemas were younger as compared to those who did not develop edemas (1.1 vs. 4.5 years [p = 0.01]), with higher potassium concentrations at T0 (4.6 vs. 4.2 mmol/L, p = 0.02) and lower serum creatinine values both at T0 (0.25 vs. 0.41 mg/dL, p < 0.01) and at T1 (0.20 vs. 0.31 mg/dL, p < 0.01). Analisis of the estimated glomerular filtration rate (eGFR) corrected by height, showed that there were no significant differences in the presence of edema (Table 2).
Bivariate analysis of the general cohort of patients based on the development or not of edema in the first 24 h of admission.
| Edema No (n = 68) | Edema Yes (n = 16) | p | |
|---|---|---|---|
| Age (years) | 4.5 (0.9−13) | 1.1 (0.5−3) | 0.01a |
| Average stay (days) | 3.0 (2−5) | 4.0 (2.2−6.5) | 0.42 |
| pH T0 | 7.36 (7.33−7.40) | 7.34 (7.27−7.38) | 0.17 |
| HCO3 T0 (mmol/L) | 23 (20−25) | 21 (20−24) | 0.60 |
| Na T0 (mmol/L) | 136 (134−138) | 137 (136−139) | 0.23 |
| K T0 (mmol/L) | 4.20 (3.80−4.80) | 4.6 (4.3−5.2) | 0.02a |
| Cl T0 (mmol/L) | 102 (100−104) | 101 (100−105) | 0.48 |
| Blood glucose T0 (mg/dL) | 96 (88−106) | 100 (85−129) | 0.59 |
| Urea T0 (mg/dL) | 22 (17.7−32) | 21 (13−27) | 0.25 |
| Creatinine T0 (mg/dL) | 0.41 (0.31−0.61) | 0.25 (0.21−0.31) | <0.01a |
| eGFR at T0 (mL/min/1.73 m2) | 110 (97.3−125) | 122.1 (99.8−171) | 0.08 |
| Kidney damage pRIFLE % | N = 6 | N = 1 | 0.43 |
| % weight gain in T1 | 0.3 (0−2.4) | 0.1 (0−5.5) | 0.90 |
| pH T1 | 7.39 (7.36−7.40) | 7.36 (7.33−7.40) | 0.28 |
| HCO3 T1 (mmol/L) | 24 (21−25) | 22 (20−23) | 0.13 |
| Na T1 (mmol/L) | 138 (137−140) | 139 (137.5−141) | 0.30 |
| KT1 (mmol/L) | 4.1 (3.9−4.3) | 4.6 (3.8−4.67) | 0.11 |
| Cl T1 (mEq/L) | 106 (104−109) | 108 (106−110) | 0.12 |
| T1 blood glucose (mg/dL) | 100 (90−113) | 99 (91−126) | 0.79 |
| Urea T1 (mg/dL) | 14 (9−20) | 10 (4−23) | 0.27 |
| T1 creatinine (mg/dL) | 0.31 (0.22−0.49) | 0.20 (0.19−0.22) | <0.01a |
| plasma T1 osmolarity | 287 (280−293) | 291 (284−296) | 0.14 |
| eGFR at T1 (mL/min/1.73 m2) | 125 (101−148) | 140 (94.4−171) | 0.19 |
| Male gender (%) | 53% | 68.8% | 0.27 |
| Diagnosis at admission | |||
| Respiratory | 13% | 37% | |
| G.I. | 35% | 12% | 0.18 |
| Infectious | 30% | 37% | |
| Others | 22% | 14% | |
| underlying pathologies (%) | 21% | 31% | 0.34 |
| DRIP RATE | |||
| 100% of BFN _ | 60% | 62% | 0.55 |
| Less than 100% of BFN | 40% | 38% |
-BFN: basal fluid needs calculated according to Holliday-Segar formula.
eGFR: estimated glomerular filtration rate using the 2009 updated Schwartz equation.
The results are expressed in medians with interquantile range (p25–p75).
There were no differences in the development of edema, in the those children with acute kidney injury at the start of fluid therapy in both groups; furthermore no differences were observed in chloride, sodium, osmolarity, glycemia, patient intake or percentage of weight gain after 24 h after fluid therapy (Table 2). The patients who had kidney damage had an average GFR of 50.8 (mL/min/1.73 m2) with a range from 23 to 80 (mL/min/1.73 m2) resolved in the following 24 h, thus the low GFR was probably of pre-renal origen due related to low oral intake.
A logistic regression study was performed whose dependent variable was the development of edema as a complication. We found that higher values of chloride in T1, after the first 24 h of fluid therapy were associated with the development of edema (OR 1.73 [1.0−3.8], p = 0.06) (Table 3), as well as higher GFR values before initiation of fluid therapy (OR 1.20 [1.1–1.5], p = 0.02).
Multivariate study for the analysis of independent risk factors in the development of edema in the first 24 h of fluid therapy. Logistic regression (dependent variable: edema).
| Edema OR (95% CI) | p | |
|---|---|---|
| Glomerular filtration rate T0 | 1.20 (1.1−1.5) | 0.02a |
| T0 rate | 0.95 (0.86−1.1) | 0.15 |
| Chloride T1 | 1.73 (1.0−3.8) | 0.06a |
| Bicarbonate T1 | 1.31 (0.87−2.77) | 0.29 |
R2: 0.66.
In the linear regression analysis, neither the drip rate nor age were associated with higher levels of chlorine 24 h after fluid therapy (Table 4).
Multivariate study for the analysis of independent risk factors that influence the chloremia analyzed 24 h after fluid therapy. Linear regression (dependent variable: Chloremia at T1).
| Chloremia (Coefficient t) | p | |
|---|---|---|
| Age | −0.11 | 0.90 |
| dripping rate | −0.55 | 0.58 |
| glomerular filtration rate T0 | −1.70 | 0.10 |
| Blood glucose T0 | −3.20 | <0.01a |
R2: 0.30.
Anova of the model. p < 0.01.
The main objective of this study was to describe the complications associated with the widespread use of isotonic fluids in pediatric hospitalization wards in stable patients. Almost 20% of the patients who received IV isotonic maintenance fluids presented edema and 16.6% hyperchloremia, these figures being much higher than those published in previous studies.5 These two main complications are probably related to the excessive administration of isotonic fluids, as discussed later.
Regarding age, there is controversy in the literature about the age at which the use of isotonic fluids is safe. The 2015 NICE guidelines8 permit its use since birth, but the American pediatric guidelines recommend its use since one month of life onwards.2 Other authors yet recommend the use of hypotonic intravenous fluids in infants under three months of age or under 10 kg11,12 probably related to immature renal function. In our study, edema appeared more frequently in younger children. The lower creatinine values in patients with edema are correlated with younger age and not with renal function, as confirmed in the multivariate study.
Regarding the rate of fluid administration, most of the previous studies on maintenance fluid therapy do not specify the amount of fluid administered or they use the Holliday and Segar formula. This formula calculates the fluid needs to metabolize daily calories, thus covering insensible losses (35%), diuresis (60%), and feces (5%) under normal conditions, that is, healthy children with normal weight and height.1,13 During hospitalization, all body losses can vary, so this formula may not reflect accurately the fluid requirements of a sick child. There are no previous studies evaluating the appropriate rate of administration in patients admitted for an acute intercurrent process, although a growing numbe of authors are wondering whether the Holliday and Segar formula could be overestimating fluid needs.1,16,17
19% of our patients presented edema after the administration of isotonic fluids. Most edemas were palpebral and on the back of the hands, without major clinical consequences, resolving in the following 24 h after the change in composition and rate of fluid therapy. The American guidelines refer to the lack of studies evaluating fluid overload, although they warn that there is a risk of fluid overload, mainly in critically ill patients, with fewer studies in patients on general pediatric wards.2,16,18 Therefore, edemas are rarely referenced in the literature, only in 3.7%.5
Isotonic fluids are distributed throughout the extracellular compartment (plasma and interstice), so an excessive supply causes an increase in interstitial fluid (edema) or arterial hypertension.1,19–21 Hypotonic fluids, having a tonicity of 70 mEq/L are distributed throughout the interstitial fluid, but also in the intracellular compartment, thus having a greater risk of hyponatremia and cellular edema.1,22,23 In this sense, if we use the same formula to calculate the fluid therapy rhythm but with isotonic fluids, we will be able to avoid iatrogenic hyponatremia but with a greater risk of producing edema and hyperchloremia probably due to an overload of sodium and chloride.
Hyperchloremia secondary to the administration of isotonic fluids has been documented in previous publications, mainly in adults admitted to intensive care.24–26 This complication has been solved with the use of balanced solutions (Plasmalyte®, Ringer®) used in surgical patients,28 but more studies are needed to analyze its safety in general pediatrics.27,28 Hyperchloremic acidosis has a risk of renal vasoconstriction and a decrease in glomerular filtration, as well as an increase in proinflammatory cytokines.29,30 In our study, hyperchloremia within the first 24 h of starting fluid therapy is a risk factor in the development of edema. Patients who presented hyperchloremia showed clinical symptoms of malaise, refusal to eat, and decreased diuresis, fundamentally, without evidence of alteration in the respiratory pattern or arrhythmias.
In our series, it is notable the high percentage of patients with hyperglycemia (46%) and hyperosmolarity (35%), probably related to the excessive administration of glucose and the administration of hyperosmolar fluids. These complications are related to the use of 5% glucose saline solution without being able to generalize to other isotonic solutions such as 0.9% saline solution. The osmolarity of these 5% glucosaline fluid s is 586 mOsm/L, but theoretically the osmolarity provided by glucose should be subtracted since it will be metabolized in the tissues (especially liver, muscle, and fat tissue), so it would not influence tonicity according to the manufacturers.1,2 However, this process requires time, and while it meanwhile, an osmotic release of antidiuretic hormone (ADH) occurs, which may explain the tendency to save free water and the appearance of edema, and also depends on the metabolism of the hospitalized patient. In our study, hyperosmolarity has not been associated with the risk of edema, but at least in theory, it could be playing a role given that plasma osmolarity is the main regulator of total body water, producing an osmotic release of ADH that would justify the appearance of edema.16 It is probable that patients who are admitted, due to their stressful situation,1 have lower needs of glucose, so we believe that these intakes should be adjusted not only to the patient's age but also to their clinical situation.
Less frequent complications were sodium changes such as hyponatremia (2.3%) in percentages similar to previous studies.4,5 Regarding the risk of hyponatremia due to fluids, it does not completely disappear with the use of isotonic fuids, and it could be related to an increase in antidiuretic hormone (ADH) secondary to stress, pain, respiratory problems or infections that are usually present during hospitalization, so we once again recommend the use of restricted isotonic maintenance fluids on admissions to general wards of pediatry.
This study has some limitations because of it is a single-center, observational study based on routine clinical practice and patients were not randomized; however, it reflects our “real life” experience. Other limitations are related to the measurement of diuresis, which was estimated subjectively by the nursing staff. The ADH values were not collected since it is not part of routine clinical practice, so their value is unknown. Finally, it should be noted that the samples collected are venous, in accordance with clinical practice in the pediatric population, but with a greater dispersion in pCO2, unlike blood samples from artery extraction, so it is possible that some acidosis are false respiratory acidosis. However, we emphasize that it is the first prospective study carried out on patients admitted to pediatric hospitalization wards.
ConclusionsThe administration of isotonic fluids is not free of complications, and they are probably related to the rhythm of administration. The youngest children have higher risks of developing edema. Close monitoring of patients receiving fluid therapy is necessary, including analytical controls in the first 24 h and adjustment of the patient's intake rate.1,11,12 Studies are needed to review fluid requirements to calculate rhythms of infusion in maintenance fluid therapy. Meanwhile, we recommendthe use of more restricted rhythms when using isotonic maintenance intravenous fluids, especially in infants.
Key concepts
- none-
The main complications of the administration of isotonic fluids in maintenance fluid therapy are edema and hyperchloremia.
- none-
These complications are mainly due to the rate of administration.
- none-
Infants are the patients with the highest risk of presenting complications associated with the use of isotonic fluids.
The realization of this work has not had funding sources from public or private entities, research or foundations.
With conflict of interestThe authors declare that they have no conflict of interest.