The regulation of mineral metabolism is achieved trough a complex interaction of hormonal factors and target organs. Before the discovery of FGF23 we believed that the regulation of serum calcium and phosphate was mainly the result of changes in PTH and vitamin D acting on bone, kidneys and intestine. Parathyroids and kidneys were responsible for the production of PTH and 1,25(OH)2D3 respectively. Presently we know that FGF23 is produced by bone so the bone is not longer just a target organ but an active endocrine organ that participate in the regulation of mineral metabolism by sending signals through FGF23. Nephrologists are knowledgeable about the regulation of calcium and phosphate otherwise it is difficult to understand and manage the disturbances of mineral metabolism that are always present in patients with CKD. Changes in mineral metabolism in CKD are now described as chronic kidney disease-mineral and bone disorders (CKD-MBD).1 The pathology derived from CKD-MBD includes not only bone abnormalities but cardiovascular disease with a devastating prevalence of vascular calcification. The severity of CKD –MBD is associated with increased mortality in CKD patients.
THE REGULATION OF SERUM PHOSPHATE
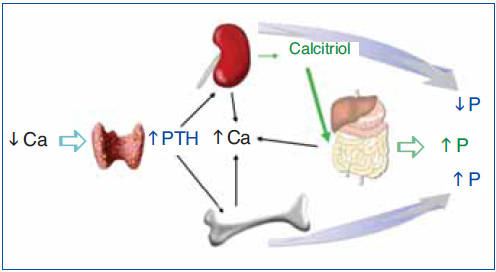
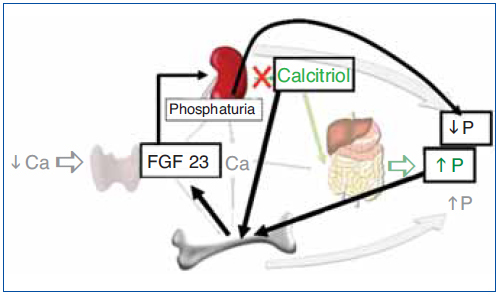
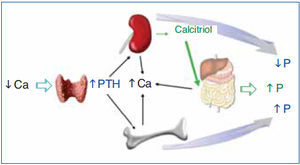
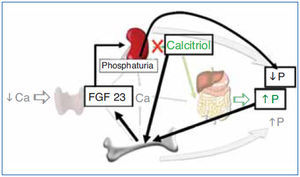
The regulation of calcium and phosphate was only partially understood until the discovery of FGF23. FGF23 increases phosphaturia a reduces the production of 1,25(OH)2D3 (Figure 1). Let's think in a situation of hypocalcemia; the parathyroids respond promptly to a decrease in serum calcium, elevated PTH acts on bone to increase the exit of calcium, but the calcium release from bone is also accompanied by the release of phosphate. The PTH acts also in kidneys increasing the tubular reabsorption of calcium so the calcium released by bone is kept in the extracellular space. The PTH produces phosphaturia so the phosphate released by bone does not build up in the extracellular space. This may not be sufficient to bring the calcium up to normal, therefore the elevated PTH stimulatesrenal production of 1,25(OH)2D3 which in turn stimulates intestinal calcium absorption. This regulatory system appears to be adequate to control serum calcium, however 1,25(OH)2D3 not only increase gut absorption of calcium but also the absorption of phosphate. It does not seem logical that a synchronized hormonal response to correct hypocalcemia had to be concluded with an excess of phosphate. FGF23 modulates the production of 1,25(OH)2D3 and the accumulation phosphate. Both high phosphate and 1,25(OH)2D3 stimulate the production of FGF23 which feeds back on the production of1,25(OH)2D3 and induces phosphaturia. Thus the presence of FGF23 enables the system to restore the serum calcium without the trouble of phosphate accumulation (Figure 2).
PRODUCTION AND ACTIONS OF FGF23
FGF23 is a 32-kDa (251 amino acid) protein produced by osteocytes and osteoblasts which makes the bone an endocrine organ that communicates with other organs involved in mineral homeostasis. FGF23 acts on its receptor complex, klotho-FGFR1, in the kidney to cause phosphaturia and to decrease calcitriol synthesis.2-5 FGF23 induces phosphaturia by suppressing the expression ofthe Na-Pi cotransporters 2a and 2c in the brush border of renal proximal tubules. FGF23 suppresses renal production of 1,25(OH)2D3 by inhibiting 1α-hydroxylase (CYP27B1) activity which produces 1,25(OH)2D3 from 25(OH)D and also by increasing 24-hydroxylase activity which inactivates the 1,25(OH)2D3.3,6 Therefore the lack of FGF23, as in the FGF23 null mouse (FGF23-/-) causes hyperphosphatemia and high levels of 1,25(OH)2D3 a situation that produces extraosseous calcification.7 The endocrine action of FGF23 is dependent upon its binding and activation of the klotho-FGFR1 complex,5 therefore the absence of klotho as in the klotho-/- mouse produces a phenotype similar to the FGF23-/-mouse, elevation of phosphate and 1,25(OH)2D3 together with calcifications. We should be aware that these FGF23-/- rodents are teaching us what has been clinically evident in uremic patient: excessive doses of Calcitriol in combination with hyperphosphatemia carries the risk of calcification.
FGF23 production by osteoblasts and osteocytes is stimulated by high dietary intake of phosphate however the mechanisms at the cellular level are unknown.8 Experiments have failed to show a direct effect of high extracellular phosphate concentration on FGF23 expression by bone cells.8 The stimulation of FGF23 production by 1,25(OH)2D3 is well defined. Liu S et al.9 showed that 1,25(OH)2D3 up regulates FGF23 expression by acting on VDR response elements of the FGF23 promoter. Interestingly Carrillo et al.10 have shown thatestrogens directly stimulate the production of FGF23.
THE INTERRELATIONSHIP FGF23-PTH
Parathyroid tissue expresses a significant amount of klotho11 and FGF23 receptor. Thus it was reasonable to anticipate an effect of FGF23 on the parathyroids. FGF23 acts on the parathyroid FGF-Klotho complex12 causing activation ofthe MAPK pathway through ERK1/2 phosphorylation and increasein early growth response 1 mRNA levels. In vivo and in vitro experiments demonstrate that FGF23 decreased PTH mRNA and PTH secretion.12-14 FGF23 also produces upregulation of parathyroid 1 alpha hydroxilase expression.13 Canalejo et al.14 investigated the effect of FGF23 on two main parathyroid receptors that inhibit parathyroid function: the calcium sensing receptor and the vitamin D receptor. In vivo and in vitro studies demonstrated that FGF23 increased gene expression and protein levels of both calcium sensing and Vitamin D receptors. Finally the same authors showed that FGF23 decreased parathyroid cell proliferation. All these results strongly suggest that FGF23 inhibits parathyroid function in normal parathyroids. The expression of FGF23 receptor and klotho in parathyroids have been investigated. Some experiments have shown that administration of FGF23 produces upregulation of parathyroid klotho,13 other authors14 observed that FGF23 produced an increase in klotho that did not reach significance. High extracellular calcium was able to increase in both parathyroid klotho and FGF receptor expression in normal parathyroid glands.14
FGF23 IN PATIENTS WITH CHRONIC KIDNEY DISEASE. THE PATHOGENESIS OF SECONDARY HYPERPARATHYROIDISM
Several publications have illustrated the important changes in FGF23 levels in patients with CKD.15-18 Some authors have shown that in early stages of CKD serum levels of FGF23 are elevated even when PTH is not significantly increased. For many years accumulation of phosphate and vitamin D deficiency were considered the key factors in the development of secondary hyperparathyroidism.18 The increase in serum PTH in CKD not only promotes urinary excretion of phosphate but also maintains serum calcium levels and stimulate the failing kidney to produce 1,25(OH)2D3. The increased production of FGF23 in CKD patients is most likely due to the increase in body burden of phosphate (not necessarily accompanied by hyperphosphatemia). FGF23 induces phosphaturia, which may explain why serum levels of phosphate are maintained in early stages of CKD. However FGF23 decreases de production of 1,25(OH)2D3 and accelerates its metabolism by augmenting 24(OH) asa activity. Thus, the decrease in 1,25(OH)2D3 seen in early CKD may be attributed not only to the decrease in renal mass but also to the early increase in FGF23.19 There is a debate about which of the two phosphaturic hormones, PTH or FGF23 increases earlier in CKD.20-22 An study by Isakova T et al.22 showed that in a group CKD patients with an average GFR of 41 ml/min had normal serum levels of calcium, phosphate and PTH, however FGF23 were already elevated and 1,25(OH)2D3 levels significantly reduced. Progressive loss of nephrons will make both FGF23 and PTH non-operative and then serum phosphate concentration will increase.
Nephrologists frequently ask whether or not it is advantageous to have elevation of FGF23. Certainly FGF23 helps to control phosphate balance but contributes to vitamin D deficiency. Furthermore recent experiments demonstrate a direct negative effect of FGF23 on the cardiovascular system.23 The fact that FGF23 is elevated indicates that the failing kidney needs the ”help” of a phosphaturic hormone able to handle the phosphate load. Therefore the increase in FGF23 implies inadequate phosphate control. In patients with CKD a better control of phosphate is associated with a decrease in FGF23.24 Another question is whether FGF23 is a clinical usefultool to assess phosphate balance in CKD patients. FGF23 levels may not reflect acute changes in dietary phosphate; however high serum level of FGF23 may reveal a long period of positive phosphate balance. Certainly, clinical studies will have to be performed to prove the usefulness of FGF23 as a marker of phosphate balance.
A considerable amount of clinical studies have shown that a high FGF23 level is independent predictor of mortality,25-27 progression of renal disease28,29 left ventricular hypertrophy,30,31 vascular dysfunction,32 renal transplant outcome33 and experimental work have shown that FGF23 causes ventricular hypertrophy directly.23
FGF23 IN ADVANCED SECONDARY HYPERPARATHYROIDISM
In dialysis patients serum FGF23 levels are markedly increased and they are positively correlated with serum PTH levels and with serum levels of phosphate.16 One may assume that the sustained accumulation of phosphate is the cause of a direct correlation between PTH and FGF23. Nevertheless, given the fact that FGF23 inhibits parathyroid function it is unexpected to observe a parallel increase in the serum concentrations of FGF23 and PTH.
Experimental work in uremic rats with secondary hyperparathyroidism revealed that administration of FGF23 did not reduce serum levels of FGF23 in uremic rats; and, in vitro hyperplastic parathyroid glands from uremic rats did not respond to FGF23. Further experiments showed that hyperplastic parathyroid glands presented low expression of both FGF receptors and klotho. This results suggests a resistance of hyperplastic parathyroid gland to the inhibitory action of FGF23.14 Similar results were obtained by other group in another rat model of renal insufficiency.34 In parathyroid glands obtained from patients with advanced secondary hyperparathyroidism Klotho and FGFR1c expression decreased significantly particularly in glands with nodular hyperplasia.35-37
FGF23 AFTER FENAL TRANSPLANT
After renal transplant many patients maintain high FGF23 levels suggesting that FGF23 may be the cause of of post-transplant hypophosphatemia with a relative vitamin D deficiency.38-40 Before transplantation FGF23 levels are very high and after kidney transplantation the excess of FGF23 acts to promote phosphaturia and suppress 1,25(OH)2D production. It is not clear why FGF23 secretion is maintained after transplantationdespite hypophosphatemia.
Conflict of interest
The authors declare potential conflicts of interest. Grants: Amgen, Abbott, Fresenius Presentation honoraria: Amgen, Abbott, Fresenius, Shire, Genzyme, Roche, Vifor Consultant honoraria Amgen, Abbott, Fresenius, Shire, Genzyme, Roche, Vifor.
Figure 1. Hormonal response to hypocalcemia.
Figure 2. Hormonal response to hypocalcemia and the role of FGF23 to maintain phosphate balance.