Introducción: En Venezuela se ha descrito una nueva variedad de nefronoptisis, denominada nefronoptisis del adolescente, la cual expresa características clínicas e histológicas similares a las otras variedades ya descritas. Sin embargo, la patogenia de esta enfermedad aún no es bien conocida. El objetivo de este trabajo fue determinar la expresión del receptor del factor de crecimiento epidérmico (EGFR) humano en las células epiteliales tubulares de pacientes con nefronoptisis del adolescente. Métodos: se estudiaron las biopsias renales de 8 pacientes con nefronoptisis del adolescente, mediante la técnica de inmunohistoquímica, para determinar la expresión renal del EGFR. Resultados: En todas las muestras la expresión del receptor del factor de crecimiento epidérmico fue negativa. Conclusión: Estos hallazgos indican una deficiencia del receptor del factor de crecimiento en los epitelios indiferenciados, lo cual podría ser uno de los factores desencadenantes del desarrollo de los quistes en la nefronoptisis.
Introduction: In Venezuela has been described a new form of nephronophthisis, called adolescent nephronophthisis, with clinical and histological findings very similar to others varieties described. However, pathogenesis in not well known. The aim of this study was to determine the expression of human epidermal growth factor receptor (EGFR) in tubular epithelial cells of patients with adolescent nephronophthisis. Methods: Renal biopsies of 8 patients with adolescent nephronophthisis were studied by immunohistochemistry to determine renal expression of EGFR. Results: In all patients, there was no expression of epidermal growth factor receptor. Conclusion: These findings indicate a deficiency of growth factor receptor in undifferentiated epithelial cells, which could be one factor in the development of cysts in nephronophthisis.
INTRODUCTION
Nephronophthisis (NP), an inherited disorder that is transmitted in an autosomal recessive manner, forms part of the group of medullary cystic diseases that lead to chronic kidney disease (CKD), usually in the second decade of life.1
A new type of this disease, adolescent nephronophthisis or NPHP3,2 was discovered in the Venezuelan population. The histological characteristics of NPHP3 are similar to those described in juvenile nephronophthisis: tubular atrophy with thickening of the tubular basement membrane (TBM) with variable thickness, diffuse interstitial fibrosis and cysts formation in the corticomedullary junction and the renal medulla.3,4
In the electron microscopy for NP, we can observe rolling, splitting, doubling, folding, thinning and thickening of the TBM.5 These findings imply that there are abnormalities in the structure and composition of the TBM, suggesting that this is the primary or fundamental defect in NP.4
Evidence from a number of laboratories has demonstrated the important role of the axis of the epidermal growth factor receptor (EGFR) in promoting epithelial hyperplasia in cystic epithelium. This results in kidney cyst formation and gradual expansion, both in autosomal dominant polycystic kidney disease (ADPKD) and in autosomal recessive polycystic kidney disease in mice and humans. Furthermore, the cystic renal epithelium presents both quantitative (overexpression) and qualitative (wrong location) alterations in the expression of one or more members of the ErbB receptors family.6
Since NP is one of the medullary cystic diseases, in this study we determined the expression of human EGFR in tubular epithelial cells of 8 patients with NPHP3 through immunohistochemical staining so as to try to explain its involvement in this pathology.
METHODOLOGY
We studied the renal biopsies of 8 patients with NPHP3, which were evaluated at the Nephrology, Dialysis and Transplant Unit of IAHULA in Merida, Venezuela, using the immunohistochemical technique. These biopsies were performed to establish the diagnosis and assess the disease progression. The average age of patients was 20.6 ± 4.0 years. There were six male and two female patients, of whom six patients presented end-stage renal disease (ESRD) and 2 had normal renal function. The renal biopsies were taken by performing micro lumbotomy. The biopsies were subsequently placed in formalin and processed for analysis using light microscopy through the conventional method of embedding them in paraffin.
The procedure used for the study of the samples has been previously described.7 Histological incisions of 3 µm were made from the paraffin-embedded kidney tissue. These were mounted on silanised glass slides, which had been dried in the oven for 24 hours at 37°C, dewaxed in xylene and then rehydrated with ethanol solutions of decreasing degrees until distilled water is obtained. The antigen retrieval was performed by placing the glass slides in a solution of 0.1% trypsin at 37°C for 15 minutes. The glass slides were washed with distilled water before being placed for 10 minutes in an endogenous peroxidase blocking agent (peroxidase blocking, Dako, Carpinteria, USA). After washing the glass slides again with distilled water, they were incubated for 10 minutes in a solution of tris-buffered saline (TBS) in order to be subjected to the protein-blocking process (protein blocking system, Dako, Carpinteria, USA). The glass slides were incubated for 20 hours with primary antibody (mouse monoclonal anti-receptor antibody of human epidermal growth factor, Clone: H11, Dako, Carpinteria, USA) at a dilution of 1:100 at 4°C in a moist chamber.
Subsequently, the samples were left at room temperature for one hour. After washing them with TBS, the glass slides were incubated using the EnVision system (Dako, Carpinteria, USA) for 30 minutes at room temperature in a moist chamber and washed with TBS for 10 minutes. We used diaminobenzidine chromogen (DAB, Dako, Carpinteria, USA) for 10 minutes and washed them with distilled water. In the end, the histological samples prepared were contrasted with Mayer’s haematoxylin, dehydrated with a series of ethanol solutions in increasing degrees and covered with microscope slides using Martex.
Normal skin tissue was used as a positive control for the main antibody, while striated skeletal muscle was used as a negative control. These control tissues were used in accordance with the recommendations of the SPC.
The material was observed using a Medilux 12 light microscope, linked to a Leica DFC 280 camera to capture and digitize images. Two independent observers who agreed on the results monitored the findings.
For the analysis of the findings, the immunostained tissue sections were assessed using a semi-qualitative scale, considering as positive the reaction of observing a brown immunoprecipitate. Scale: Negative (0, no staining), weak intensity (+1), moderate intensity (+2) and strong intensity (+3).
RESULTS
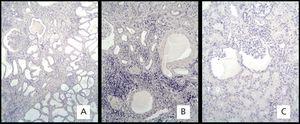
The NPHP3 diagnosis was made based on the family history, clinical histopathological characteristics and molecular genetics. The patients belonged to the above-mentioned group.2 The histological lesions were very similar to those previously described in the literature.3 Cysts were observed in 5 of the 8 cases. The size and number of the cysts varied, and they were lined with low simple cubic epithelium, with irregular thickening in the TBM. In some cysts, we observed micropapillae, which were lined with hyperplastic epithelium. All the patients’ biopsies showed diffuse interstitial fibrosis and tubular atrophy characterised by a thick and folded TBM. In one case, there was fibromuscular thickening of the arterial walls.
In all cases, we observed glomeruli with retraction of the capillary tuft, cyst-like dilation of the urinary space and concentric periglomerular fibrosis, primarily in patients with ESRD.
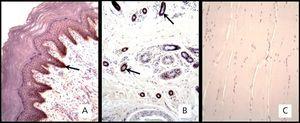
Regarding the immunohistochemistry, the positive skin test showed brown precipitate with +3 intensity in the epidermal epithelial cells and sweat glands (Figure 1A and 1B). The intensity in the negative control in striated skeletal muscle was 0 (Figure 1C). The expression of the EGFR in the biopsies of the 8 patients was 0 (Figure 2).
DISCUSSION
Growth factors have been little studied in NP. In the literature, there is only one report of a study on a 13-year-old patient with this disease, whose renal biopsy was examined using immunohistochemistry and was positive for hepatocyte growth factor (HGF), expressed in the epithelial cells of dilated tubules. This finding allowed the authors to suggest that this factor could be involved in the formation of cysts in this disorder.8
Cystogenesis has been studied extensively in ADPKD. It has been proved in vitro that the epidermal growth factor (EGF) has a mitogenic effect on the cystic tubular epithelium. In addition, the number of EGF receptors was increased, a fact that is regarded as a proliferative factor in this disease. In addition, infantile murine polycystic disease studied in C57BL/6J-cpk mice has shown that the relative lack of EGF may contribute to the development of cysts in the collecting tubule, which are caused by a delayed epithelial maturation.9
NP and its associated disorders are considered as ciliopathies, since all the products of the NPHP genes are expressed in the primary cilia, similar to the proteins of polycystic kidney disease.10
However, NP has given rise to an alteration in the structure and composition of the TBM, the components of which form a specialised extracellular matrix (ECM) that participates in normal renal tubulogenesis. It is likely that during nephrogenesis, the molecular defects in the ECM may be involved as some of the possible pathogenic factors of this hereditary tubular disorder.11 Furthermore, several reports on ADPKD animal models suggest that abnormalities in the EGFR axis are a common cellular expression that occur as a result of a diverse number of primary mutations.6
EGFR expression was negative in all the patients with NPHP3. This allows us to consider the possibility of a deficiency in the effect of EGF on the tubular epithelium. It is known that this factor is one of the EGFR ligands, which promotes cell differentiation in immature organs, suggesting that perhaps the presence of an undifferentiated tubular epithelium determines the development of cysts in this disease.
In this regard, we conclude that EGF probably acts as a cell differentiation factor, while the lack of its receptor expression in nephronophthisis constitutes indirect evidence of deficiency or absence of the trophic effect of EGF on epithelial cells. This is demonstrated through the development and maintenance of an undifferentiated epithelium, thus affecting the formation of cysts. We believe that this could be one of the factors involved in cystogenesis, and further research is required to elucidate the mechanisms involved in the pathogenesis of this disease.
Financing
The Council of Scientific, Humanistic and Technological Development of the Los Andes University (CDCHT-ULA) in Venezuela funded this project. Code M-801-04-07-A.
Figure 1. . Immunohistochemical marking with mouse monoclonal anti-receptor antibody of human epidermal growth factor.
Figure 2. Immunohistochemical marking with mouse monoclonal anti-receptor antibody of human epidermal growth factor.