Home hemodialysis (HHD) with low-flow dialysate devices has gained popularity in recent years due to its simple design, portability, and ability to provide greater freedom of movement for our patients. However, there are doubts about the adequacy that this technology offers, since it uses monitors with low-flow bath and lactate. The aim of this study was to demonstrate the clinical benefits of low-flow HHD with the NxStage System One® recently introduced in Spain. We present the results of an observational, retrospective cohort study that included the first patients who started short daily HHD with this device in 12 Spanish centers. We analyzed the evolution of 86 patients at 0, 6 and 12 months, including data related to prescription, and evolution of biochemical parameters related to dialysis dose, anemia, mineral-bone metabolism; evolution of residual renal function, medication usage, and causes of withdrawal during the followup. We were able to demonstrate that this NxStage System One® monitor, in patients with HHD, have provided an adequate dialysis dose, with optimal ultrafiltration rate, with improvement of main biochemical markers of dialysis adequacy. The usage of this technique was associated to a decrease of antihypertensive drugs, phosphate binders and erythropoietin agents, with very good results both patient and technique survival. The simplicity of the technique, together with its good clinical outcomes, should facilitate the growth and utilization of HHD, both in incident and prevalent patients.
La hemodiálisis domiciliaria (HDD) con monitores de bajo flujo de líquido de diálisis ha ganado popularidad en los últimos años gracias a su sencillez de diseño, portabilidad y capacidad de desplazamiento. No obstante, existen dudas respecto a la adecuación que este tipo de técnica ofrece, pues utiliza monitores con baño a flujos bajos y lactato. El objetivo de este estudio fue demostrar los beneficios clínicos de la HDD con el monitor NxStage System One® introducido recientemente en España.
Presentamos los resultados de un estudio observacional, retrospectivo que incluyó de manera no seleccionada a los primeros pacientes con HDD corta mediante este monitor en 12 centros en España. Se analizó la evolución clínica de 86 pacientes a 0, 6 y 12 meses, incluyendo datos relacionados con la prescripción, evolución de parámetros analíticos de dosis de diálisis, anemia, metabolismo óseo-mineral, evolución de la diuresis residual, utilización de fármacos y datos relacionados con permanencia en la técnica, y causas de salida a lo largo del seguimiento. Pudimos demostrar que este monitor proporcionó una adecuada dosis de diálisis, con tasa óptima de ultrafiltración, con mejoría de los principales marcadores bioquímicos de adecuación en diálisis. El uso de esta técnica se asoció con una disminución de antihipertensivos, captores del fósforo y agentes eritropoyéticos, observándose, además, muy buenos resultados de supervivencia tanto del paciente como de la técnica. La sencillez de este monitor unida a sus buenos resultados clínicos debería facilitar el crecimiento y utilización de la HDD, tanto en pacientes incidentes como prevalentes.
There is increasing evidence about the multiple benefits that haemodialysis (HD) with more intensive dialysis regimens can provide to patients on renal replacement therapy (RRT).1–7 However, these more frequent or longer dialysis regimens are difficult to implement in a health centre due to logistics aspects, infrastructure, personnel and costs.8 By contrast, home haemodialysis (HHD) provides an optimal environment to perform this type of therapy,5,8–11 and is currently a more effective and efficient alternative to the conventional HD. Compared with in-centre HD, HHD is associated with longer survival and better quality of life, and it is also a cost-efficient technique that enhances patient autonomy.6,8,10,12–14
Since March 2014, the NxStage System One® machine has been available in Spain for the treatment of HD patients. This system is specifically designed for HHD, and is smaller and simpler than conventional machines. In addition, it does not require a water treatment equipment for the production of dialysis fluid, which has allowed it to establish itself as the leading transportable dialysis machine available in Spain.14 However, as a portable system, it requires a low flow of dialysis fluid (maximum of 200ml/min), raising the question of whether this machine is capable of achieving an adequate dialysis dose and meeting the dialysis objectives recommended by current guidelines.15,16
For this reason, we (a group of nephrologists) set out to review the progression of the first group of patients in Spain treated with this system and analyse the results. The aim of this study was to describe and demonstrate the clinical benefits of daily, short low-flow HHD using the NxStage System One® machine, recently introduced in Spain, including short-term biochemical outcomes and longer-term clinical outcomes.
Material and methodsStudy designA retrospective observational study that included, in an unselected manner, the first patients who started short HHD using a machine with a low dialysate flow: NxStage System One® (Fresenius Medical Care, Germany) in 12 hospitals in Spain. The sites were: Hospital General de Castellón, Hospital Lucus Agusti, Hospital de Teruel, Hospital General de Valencia, Fundación Puigvert, Hospital de Poniente, Hospital Universitario General de Asturias, Hospital Virgen de las Nieves, Hospital de Burgos, Hospital Universitario Dr. Peset, Hospital Universitario La Paz and Complejo Hospitalario de Navarra. Those patients who started HHD with a system other than the NxStage System One® were excluded.
The primary objective of the study was to retrospectively analyse clinical and analytical parameters related to the dialysis adequacy of patients on HHD treated with this system at 0, 6 and 12months. A secondary objective was to analyse the long-term clinical progression of the patients, including the period of time on the HHD programme, discontinuation due to transplant, switch to in-centre HD, and death. The patients started HHD between 2014 and 2016, and were followed up until 31 October 2020.
Description of the dialysis techniqueThe NxStage System One® is a portable HD machine that uses an ultrapure dialysis fluid, with lactate buffer, at low flow (150-200ml/min), inverting the ratio between the flow of dialysate and the usual blood flow in conventional dialysis machines.17 It does not require the equipment of a water treatment or modification of the electricity supply at the house. The system allows for the use of dialysis fluid in pre-mixed 5-litre bags, or for the dialysis fluid to be produced on-site at the patient's home, after mixing a concentrate with running tap water ("PureFlow SL") without the need for a reverse osmosis system.14 With the two methods, the dialysate flow is 150−200ml/min, while the composition of the dialysis fluid obtained is sodium 140mEq/l, potassium 1–2mEq/l, calcium 3mEq/l, magnesium 1 mEq/l, lactate 40−45mEq/l, chloride 100mEq/l and glucose 1g/l. The usual blood flow used is 300−450ml/min. The system uses a high-flux polysulfone filter with a surface area of 1.6m2. In vitro dialyser clearance data, with a blood flow of 400 to 500ml/min and a dialysate flow of 200ml/min, have been reported as follows: urea 196ml/min, creatinine 184ml/min and Vitamin B12 145–150ml/min.18 Prior to starting HHD, all patients were instructed at their centre on the HHD technique, including the procedure for fistula puncture or central venous catheter connection, how to programme the machine, and problem solving, for a variable period of training. Although the NxStage System One machine can perform haemodialysis or haemofiltration, depending on whether the dialysis fluid is used as a dialysis bath or as replacement fluid,17 all patients included in the study received haemodialysis exclusively.
Description of the variables and data collectionData collection was carried out during 2017, using a standardised digital form in Excel, which included demographic, clinical and analytical parameters, at the beginning of the technique, and at 6 and 12months. The data was anonymously handled and was voluntarily transferred from the different hospitals.
Clinical data included, among others, the cause of kidney disease, time on renal replacement therapy, residual diuresis, duration of training, type of vascular access and puncture technique. The analytical parameters measured were blood levels of haemoglobin, calcium, phosphorus, albumin, bicarbonate, potassium, β2-microblogulin and C-reactive protein. The dialysis dose in each session was estimated using the balanced Kt/V (eKt/V), while the weekly dialysis dose was estimated using the standard Kt/V (Kt/Vstd), following previously described formulas.18–20 According to the KDOQI guidelines, a minimum Kt/Vstd of 2.1 per week was recommended.15 Other dialysis-related parameters collected were ultrafiltration rate, dry weight, number of sessions and hours of dialysis per week, as well as the volume of dialysis fluid used at 0, 6 and 12months. Regarding concomitant treatment, the number of daily antihypertensives and phosphate binders used, the weekly dose of erythropoietic agents, and the proportion of patients receiving intravenous iron treatment were collected. Darbepoetin alfa units were converted to epoetin-equivalent units by multiplying by 200.
Additionally, the centres collected data on long-term clinical outcomes, including kidney transplantation, switch to in-centre HD, and death. The patients started HHD between 2014 and 2016, and were followed up until 31 October 2020.
Statistical analysisDescriptive techniques were used to summarise the characteristics of the patients and the HD regimens prescribed. Quantitative variables were presented as means±SD or as medians with interquartile ranges, depending on whether they presented normal distribution. Qualitative variables were presented as frequency and percentage of patients by category.
Linear mixed-effects models were used to assess changes from baseline in clinical, laboratory parameters and the use of medication. For each parameter, the statistical significance of the linear trend in the measured times was evaluated. The test was derived from a linear mixed model of the parameter back in time, with random effects (intercept and slope) for each patient and centre. Covariate adjustment was performed for the parameter baseline level as fixed effects, and patient and centre as random terms. With this method, not only the baseline value of each parameter was considered, but also the dependent (and correlated) nature of the variables repeated over time in each patient.
Using survival curves, we estimated the cumulative incidence of kidney transplantation, switch to in-centre haemodialysis, and survival at five years after starting HHD or on 31 October 2020.
Analyses were performed with R software (version 4.0.3; The R Project for Statistical Computing, Vienna, Austria).
ResultsPatient characteristicsA total of 86 patients were included, with a mean age of 52.6±13.8 years. Table 1 shows the demographic characteristics of the patients at the start of the technique. In total, 67% of the patients were men. Almost a third of the patients (28%) had glomerulonephritis as the primary disease diagnosis, followed by 14% with diabetic nephropathy. Most of the patients (n=35; 41%) had received conventional HD (3 days/week) in the centre, while 18 patients (21%) had been treated for advanced chronic kidney disease (ACKD) and 19 patients (22%) had been on peritoneal dialysis. Approximately 10% (n=9) of the patients had undergone transplantation. The previous mean time on renal replacement therapy was highly variable, 18 months (IQR: 3−57). Over half of the patients (55%) self-dialysed using a catheter. Of the patients who self-dialysed with a fistula, 85% used the buttonhole puncture technique, and of these, 23% by self-puncture. No patient self-dialysed through a prosthetic fistula.
Patient characteristics (n=86).
| Value | |
|---|---|
| Mean age (years) | 52.6±13.8 |
| Male gender; (n, %) | 58 (67%) |
| BMI (kg/m2) | 25.8±4.4 |
| Baseline disease (n, %) | |
| Nephroangiosclerosis | 8 (9%) |
| Diabetic nephropathy | 12 (14%) |
| Glomerulonephritis | 24 (28%) |
| Polycystosis | 8 (9%) |
| Interstitial | 5 (6%) |
| Unknown | 13 (15%) |
| Other | 16 (19%) |
| Charlson comorbidity index | 4.2±2.2 |
| Origin (n, %) | |
| ACKD | 18 (21%) |
| Conventional HD (3 days/week) | 35 (41%) |
| Intensive HD (5 days/week) | 5 (6%) |
| PD | 19 (22%) |
| Transplant | 9 (10%) |
| Previous mean time on dialysis (months) | 18 (3-57) |
| Included on the transplant waiting list (n, %) | 48 (56%) |
| Travels with the NxStage® machine (n, %) | 33 (38%) |
| Training time (average number of sessions) | 25.3±13.4 |
| Vascular access type (n, %) | |
| Catheter | 47 (55%) |
| Native fistula | 39 (45%) |
| Prosthetic fistula | 0 |
| Fistula cannulation technique (n, %) | |
| Rope ladder | 6 (15%) |
| Button hole | 33 (85%) |
| Fistula self-puncture (n, %) | 9 (23%) |
Unless otherwise specified, quantitative variables are expressed as means±SD.
ACKD: advanced chronic kidney disease; PD: peritoneal dialysis; BMI: body mass index.
Most patients were prescribed five or six sessions per week (Table 2), such a percentage remained constant throughout the follow-up. Regarding the hours of dialysis per session, the majority of patients self-dialysed between 2.5 and 2.9h per session from the start and throughout the follow-up. Although an upward trend was observed in the proportion of patients self-dialysing for 2–2.4h/session, to the detriment of patients self-dialysing for more than 3h/session, these changes were not significant (p=0.16). Similarly, the weekly hours of dialysis remained unchanged throughout the first year, being greater than 14h per week. Most patients (65–76%) performed sessions with 30l of dialysate per session both at 0 months, and at 6 and 12months, without observing significant changes in the type of dialysate used. The use of heparin also remained stable, being necessary in about a third of the patients throughout the study.
Evolution of the prescribed dialysis regimen and dialysis dose reached.
| Baseline (n=86) | 6m (n=73) | 12m (n=60) | p | |
|---|---|---|---|---|
| Sessions/week (%) | ||||
| 4 | 2% | 3% | 3% | 0.53 |
| 5 | 47% | 42% | 48% | |
| 6 | 49% | 52% | 59% | |
| 7 | 2% | 3% | 0% | |
| Hours/session (%) | ||||
| 2.0−2.4 | 2% | 5% | 11% | 0.16 |
| 25−2.9 | 68% | 73% | 78% | |
| 3.0−3.4 | 28% | 19% | 8% | |
| >3.5 | 2% | 3% | 3% | |
| Hours/Week | 14.7±2.5 | 14.6±2.5 | 14.0±1.8 | 0.71 |
| Type of dialysate (%) | ||||
| Pre-mixed bags | 19% | 20% | 35% | 1.0 |
| Concentrate (PureFlow®) | 81% | 80% | 65% | |
| Litres dialysate/session (%) | ||||
| 20 | 6% | 8% | 3% | 0.89 |
| 25 | 17% | 17% | 30% | |
| 30 | 76% | 74% | 65% | |
| >35 | 1% | 1% | 2% | |
| Heparin use (%) | ||||
| None | 60% | 60% | 67% | 0.43 |
| Low molecular weight | 34% | 34% | 27% | |
| Not fractionated | 6% | 6% | 6% | |
| PRU (%) | 51±11 | 51±10 | 52±9 | 0.52 |
| Balanced Kt/V | 0.69±0.24 | 0.68±0.18 | 0.69±0.18 | 0.88 |
| Standard Kt/V | 2.8±0.6 | 2.8±0.5 | 2.8±0.4 | 0.71 |
| Ultrafiltration volume (l) | 0.965±0.630 | 0.955±0.649 | 0.865±0.525 | 0.90 |
| Ultrafiltration rate (ml/kg/h) | 5.1±3.3 | 5.2±3.5 | 4.6±2.5 | 0.99 |
Unless otherwise specified, quantitative variables are expressed as means±SD.
PRU: percentage reduction of urea.
Details of the dialysis dose reached are shown in Table 2. Using low-flow HHD, the mean Kt/Vstd was 2.8 at 0, 6 and 12months. No significant changes were observed in the dialysis dose estimated by the percentage reduction of urea (PRU) and eKt/V throughout the course of treatment. The mean ultrafiltration volume was less than 1l/session throughout the study, with a mean ultrafiltration rate of 4.96ml/kg/h. The dialysis dose reached was similar among patients who used pre-mixed bags and those who used concentrate (PureFlow®) as dialysate (Table 3).
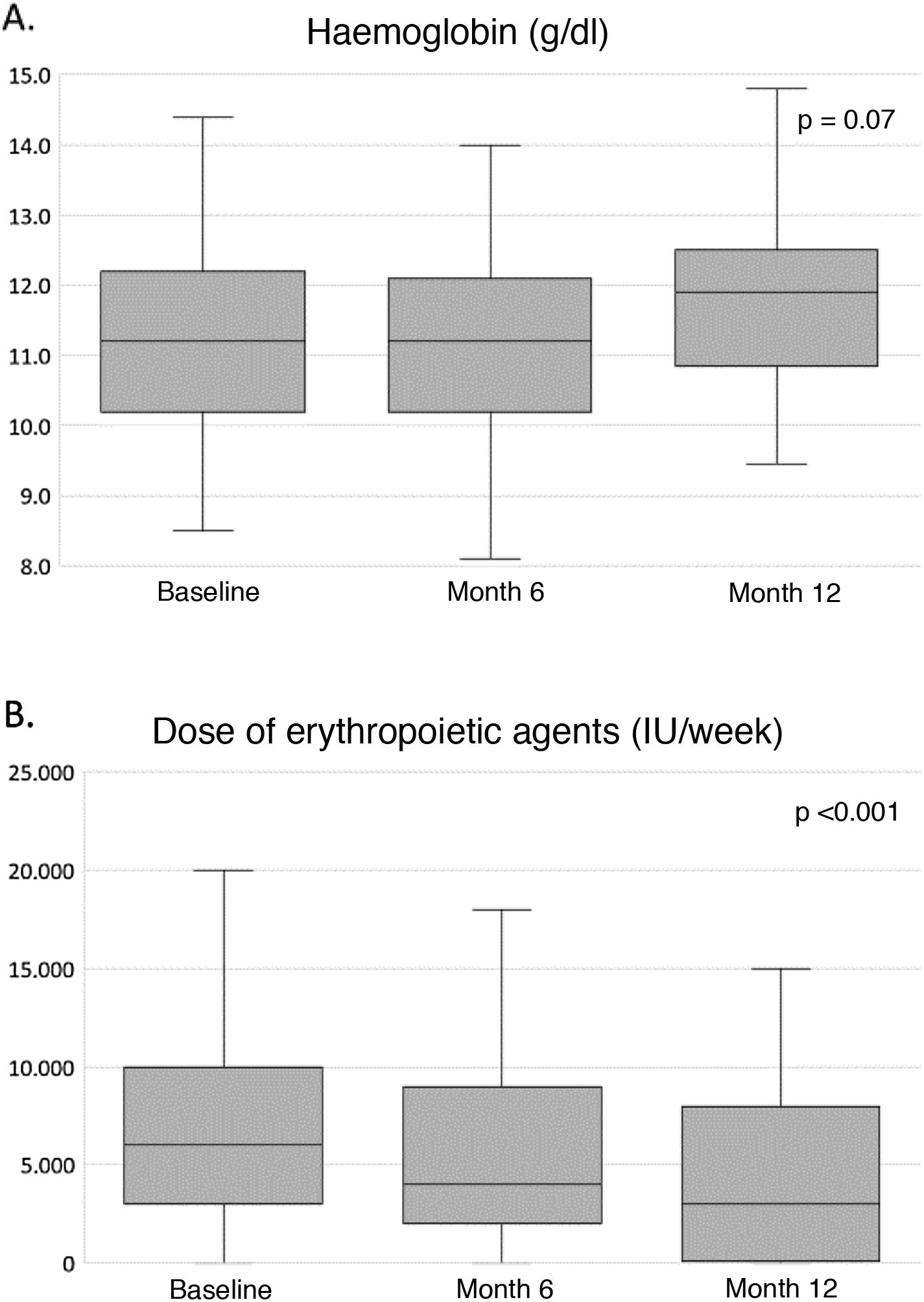
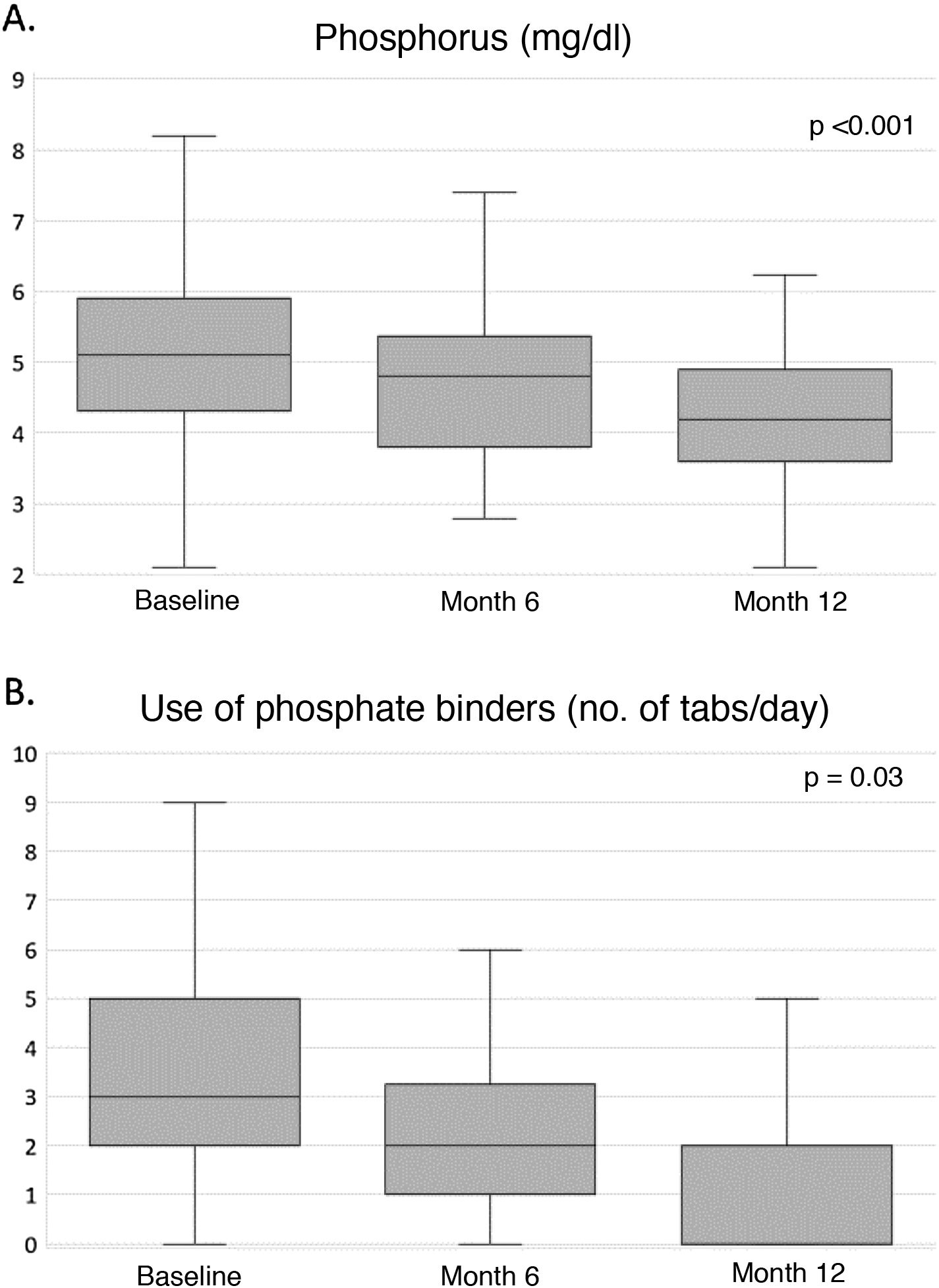
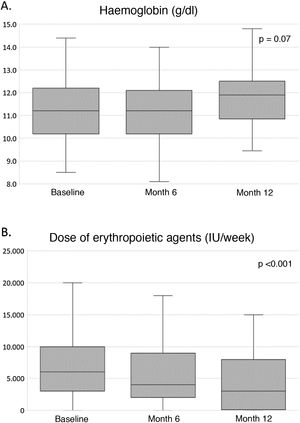
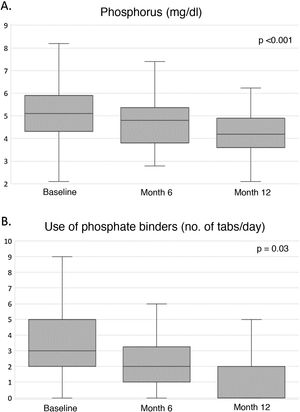
Clinical and analytical parametersTable 4 shows the evolution of the parameters related to nutrition, anaemia, inflammation, β2-microglobulin, mineral bone metabolism, electrolytes and residual diuresis during the first year of HHD treatment. Throughout the study, an improvement in nutritional parameters was observed, with dry weight and albumin levels increasing significantly from the sixth month until the end of the study. Regarding haemoglobin levels, a non-significant upward trend was observed (Fig. 1A), while the inflammatory state, estimated by the levels of C-reactive protein and β2-microglobulin, remained unchanged throughout the course of treatment. Calcium and phosphorus levels decreased significantly from month 6, remaining within normal ranges at month 12 (Fig. 2A). Potassium levels were also significantly reduced, albeit modestly, throughout the follow-up, with serum bicarbonate levels remaining stable at around 24mEq/l throughout the study (Table 4). Regarding residual diuresis, it was observed a significant decrease in the mean volume from 1,000ml/day at the beginning of the study to 600ml/day at the end of it. Although this result was expected, it is worth mentioning that more than half of the patients (n=60) maintained residual renal function at the end of follow-up.
Evolution of the clinical and biochemical parameters of the patients (n=86).
| Baseline (n=86) | 6m (n=73) | 12m (n=60) | p | |
|---|---|---|---|---|
| Nutrition | ||||
| Albumin (g/dl) | 394±0.4 | 4.15±0.4 | 4.0±0.44 | 0.01 |
| Dry weighta (kg) | 69.0 (60.3−81.3) | 72.3 (60−81.1) | 71.8 (66.8−81.0) | 0.007 |
| Anaemia | ||||
| Haemoglobin (g/dl) | 11.3±1.4 | 11.2±1.3 | 11.8±1.4 | 0.07 |
| Inflammation | ||||
| C-reactive proteina (mg/l) | 3.7 (1.7−12.0) | 3.8 (1.3−8.5) | 4.1 (2.1−7.2) | 0.75 |
| β2-microglobulin (mg/l) | 23.1±11.3 | 22.7±11.6 | 21.3±7.2 | 0.15 |
| Bone mineral metabolism | ||||
| CaAlb (mg/dl) | 9.7±1.4 | 8.9±0.8 | 9.1±0.8 | 0.002 |
| Phosphorus (mg/dl) | 5.3±2.0 | 4.7±1.2 | 4.1±1.0 | <0.001 |
| Electrolytes | ||||
| Potassium (mEq/l) | 4.5±0.8 | 4.4±0.7 | 4.2±0.8 | 0.03 |
| Bicarbonate (mEq/l) | 23.8±3.39 | 24.4±3.11 | 24.0±2.81 | 1.0 |
| Residual diuresisa (ml/dl) | 1,050 (200−2,800) | 1,000 (2,096−1,600) | 600 (125−1,500) | <0.001 |
CaAlb: albumin-corrected serum calcium.
Table 5 shows the use of antihypertensives, erythropoiesis stimulants, intravenous iron therapy and phosphate binders at 0, 6 and 12months. The median number of antihypertensive agents was significantly reduced by half during the course of treatment. Similarly, the equivalent dose of erythropoiesis-stimulating agents was also reduced by half, from 6,000 (3,000−10,000) to 3,000 (1,500−8,000) IU/week (Fig. 1B), without changing haemoglobin levels or the use of intravenous iron. Finally, the median number of daily phosphate binder tablets was significantly reduced from three to two daily tablets from the first six months to the end of the study (Fig. 2B).
Evolution of pharmacological treatment.
| Baseline | 6m | 12m | p | |
|---|---|---|---|---|
| (n=86) | (n=73) | (n=60) | ||
| Antihypertensivesa (drugs/day) | 2 (1−3) | 1 (0−2) | 1 (0−2) | <0.001 |
| Erythropoiesis stimulatorsa (IU/w) | 6,000 | 4,000 | 3,000 | <0.001 |
| (3,000−10,000) | (2,000−8,500) | (1,500−8,000) | ||
| Intravenous iron (%) | 62% | 54% | 54% | 0.63 |
| Phosphorus bindersa (tabs/day) | 3 (2−5) | 2 (1−3) | 2 (0−4) | 0.03 |
tabs/day: tablets per day; IU/w: equivalent International Units per week.
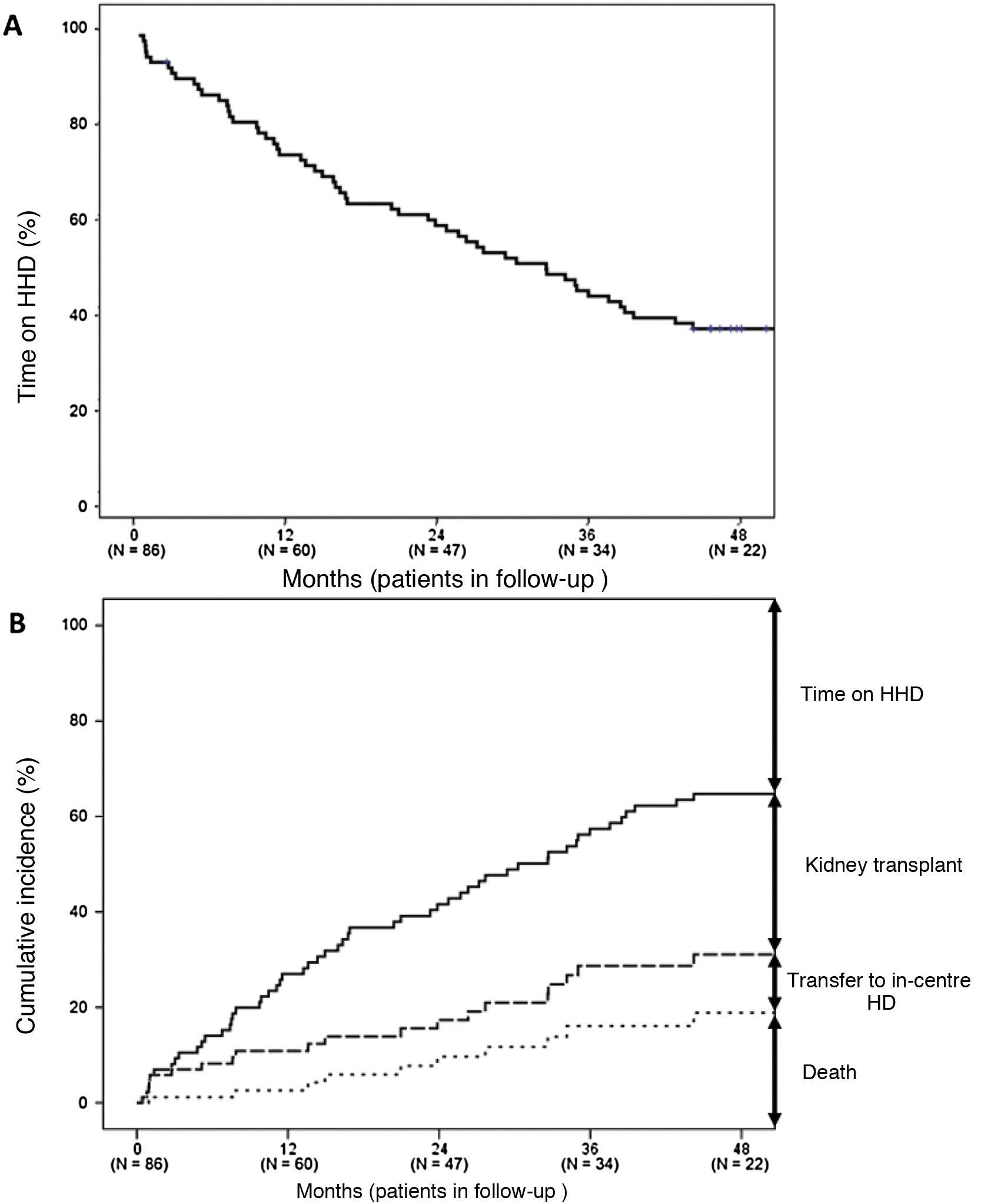
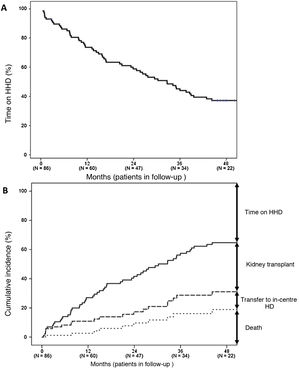
After a median follow-up of 30 (11–51) months, 24 patients (28%) remained on HHD (Fig. 3A), a transplant (n=38, 44%) was the main reason for stopping HHD (Fig. 3B). Thirteen patients (15%) died, with cardiovascular disease (ischaemic heart disease, n=4; stroke, n=1; ruptured aortic aneurysm, n=1; sudden death, n=1) and neoplasms (n=3) being the main causes. Other causes of death included intestinal perforation (n=1), abdominal infection (n=1) and cardiac arrest during an interventional procedure (n=1). Meanwhile, 11 patients (13%) returned to in-centre HD due to patient stroke (n=3), patient fatigue (n=2), caregiver fatigue (n=2) and other causes (n=4).
The long-term rates of transplantation, switch to in-centre HD and death were 17.3, 5.0 and 5.9 events per 100 patients/year, respectively.
DiscussionHHD provides multiple benefits to our patients.1–6 However, the prevalence of patients in this treatment modality is still very low, both globally and nationally, being less than 2% of dialysis patients on in our setting.21–23 Various reasons hinder the development of HHD, including economic, organisational and logistical reasons, as well as barriers related to health personnel, the patient or the caregiver.24,25 Aware of the versatility and benefits that HHD confers, more and more nephrologists opt for this treatment modality,26,27 offering it more frequently in the treatment of advanced chronic kidney disease (ACKD) and even at times when a patient requires a change in their treatment modality.28,29 The technological advances in recent years have made it possible to simplify HHD machines, thereby reducing some barriers, undoubtedly contributing to their greater use and acceptance by patients.30,31 This study describes, for the first time, the clinical results of the first patients treated in Spain with the NxStage System One® transportable home haemodialysis machine.
This machine provides the advantage of obtaining dialysis fluid either through an acidic concentrate using tap water (without the need for a reverse osmosis system), or through the use of ultrapure fluid bags with lactate. From the patient's perspective, this feature makes it relatively easy to learn and handle, as well as offering the possibility of being able to travel with it. However, from a medical perspective, these characteristics are what make many professionals doubt the ability of this machine to achieve an adequate dialysis dose and meet the dialysis goals recommended by current guidelines.15,16 We hope this publication will be of interest to provide evidence about the advantages of daily short HHD with low-flow haemodialysis.
Analysis of our cohort shows that the mean age of patients was 53 years, approximately a decade lower than the mean age of prevalent patients in many European countries,32 but the age was similar to that of HHD patients treated in the United States.33 This relative youth of the patients, together with the low prevalence of diabetes as a primary aetiology of CKD, could lead us to think that HHD is only suitable for young and healthy patients. In this regard, it should be emphasised that the age range was from 18 to 84 years. Most patients came from in-centre HD or following graft failure, with a median stay on RRT of almost four years and a mean Charlson index of 4.2. Additionally, BMI varied between 17.3kg/m2 (low weight) and 44.8kg/m2 (extreme obesity). All these data suggest HHD may be an appropriate RRT modality for a wide variety of patients, including those with greater comorbidity, who are precisely the patients that can benefit most from more frequent haemodialysis sessions.11
We observed that most of our patients on HHD were prescribed a frequency of 5–6 days per week, between 2.5 and 3h per session, with a volume of dialysis fluid of 30l per session, representing a bath flow of 150 to 200ml/min, compared to the usual 500−700ml/min of conventional machines. As a whole, the patients accumulated an average of more than 14h of dialysis per week, while the vast majority of in-centre HD patients in Spain and other European countries did not accumulate more than 12h per week.34 This intensive dialysis regimen allowed to achieve a dialysis dose estimated by Kt/ V std of around 2.8, well above the 2.1 established as the minimum objective by the KDOQI guidelines,15 and similar to those obtained in other European registries,35 demonstrating that low-flow dialysis can achieve high saturation of the dialysis bath and adequate clearance of small molecules.11,35 At this point, it should be noted that no results were collected on residual renal function (except volume of diuresis), which could further improve these results. It is important to remember that adequate dialysis is a broader concept than the simple dialysis dose measured by Kt/V,11 being defined as the RRT that meets the requirements of being effective and sufficient, achieves good tolerance, improves the quality of life and prolongs the survival of patients, also encompassing various aspects such as adequate control of anaemia, bone mineral metabolism, nutritional status, blood pressure, and electrolyte and acid-base balance.36 Most biochemical parameters improved slightly but significantly during the first year of treatment. Haemoglobin levels remained stable with less need for erythropoietin-stimulating doses throughout follow-up. The patients maintained adequate control of bone mineral metabolism, with a slight but significant decrease in calcium levels, despite having only one choice of calcium concentration in the bath. More interesting was the significant decrease in phosphate levels and the use of phosphate binders throughout the follow-up, showing that there is a good correlation between the increase in weekly frequency and time, with respect to serum phosphate control. Regarding the parameters related to nutrition, it is worth emphasising the increase in albumin and dry weight figures during the follow-up time. Data reinforces the idea that by prescribing more frequent sessions, there are fewer diet restrictions, appetite increases, and we can see how nutritional parameters improve throughout follow-up,37 as has been shown in other HD regimens that are alternatives to the standard in-centre HD of 4h, 3 days a week.38,39
When analysing the changes in the prescribed technique throughout the follow-up in terms of dialysis frequency, we observed an increase (albeit not significant) in the proportion of patients with six sessions per week, with a parallel decrease in the duration of the sessions, as well as in the volume of dialysate used per session. The authors speculate that this trend is the result of the experience accumulated during the first year by both the patient and the prescribing nephrologist regarding the benefits of more frequent and shorter dialysis. While the patient may perceive a better tolerance to dialysis with less post-dialysis fatigue syndrome, as well as fewer dietary restrictions, the clinician may observe an improvement in various relevant parameters, such as control of fluid overload, blood pressure, nutritional and mineral bone metabolism parameters, improvements previously described with more frequent dialysis regimens.1–4,9,12
Regarding the preparation of the dialysis fluid used, the PureFlow® system was the most used method, as previously described,12 although we observed an upward trend in the percentage of patients who used pre-mixed bags. Several patient-related factors may explain this increase, including ease of use (the nephrologist determines the number of bags the patient needs, and the patient simply hangs and connects the bags for treatment) and flexibility for travel (bags of pre-mixed dialysate are portable along with the machine),17 determining aspects for patients to opt for this dialysis modality.40
At the beginning of this study, most of us nephrologists had some concerns regarding the composition of the fluid with this system, due to the fact that bags of pre-mixed fluid were used with a composition of electrolytes and acid base that was very different from the usual composition with traditional HHD treatments, particularly with a lower potassium composition than usual, and especially due to the use of lactate instead of bicarbonate. We were able to verify that throughout the follow-up both potassium and bicarbonate values remained within normal range. It should be emphasised that this system uses low flows of dialysis fluid, so the electrolyte gradients are not as marked, compared to conventional systems.
As expected, with the prescription of frequent sessions, ultrafiltration rates remained low, averaging around 5ml/kg/h throughout the study, and well below the threshold of 10ml/kg/h. The authors speculate that this low ultrafiltration rate undoubtedly has repercussions on the safety and good haemodynamic tolerance of these patients, with important benefits in terms of cardiovascular safety and quality of life, both in the short and long term, given that multiple studies have correlated a low ultrafiltration rate, not only with better survival, but also with a shorter post-dialysis recovery time.41–43
Closely related to a low volume of ultrafiltration, we also observed a significant reduction in the number of hypotensive drugs used throughout the follow-up, previously described with the use of short daily HHD and other intensive HD regimens.3,12,44,45 Although we did not collect the longitudinal changes in blood pressure due to the variability of these values according to the time of measurement, the significant decrease in the use of antihypertensives was, without a doubt, another added benefit that we observed in our study, as expected, when prescribing a frequent dialysis schedule.12 Another datum related in part to the increased frequency and rate of ultrafiltration is residual diuresis. As discussed in the results, and as expected, the mean volume of diuresis decreased throughout the follow-up. However, it is interesting to mention that more than half of the patients (n=60) maintained a mean volume of 600ml at the end of the study. Being a retrospective study, we had the limitation of not having other analytical results related to this aspect, which leaves an interesting area of analysis for future studies to more clearly assess the influence of daily dialysis and its effect in relation to the preservation of residual renal function.
Regarding training time, we confirmed that with this system, patients required an average of 25 sessions, compared to HHD training with conventional systems, which require about 30 sessions per patient.46 It is important to mention that more than half of the patients had a catheter as vascular access, a fact that may influence the duration of the training, since learning with cannulation of an arteriovenous fistula in some cases may be more difficult. The training time according to vascular access, as well as problems related to it, are data that have not been analysed in this study, and are data that are undoubtedly of interest for analysis in future research.
When analysing survival of the technique, we observed very good results, with a relatively low switch to in-centre HD rate, lower than that previously described with this technique,12 with a transplant being the main cause for stopping HHD for our patients. In contrast, the crude mortality rate was relatively low (5.9 per 100 patients/year), 33% lower than the average for the Spanish population on HD aged between 45 and 64 years (8.9 per 100 patients/year).23 Although this high rate of transplantation, as well as the low mortality, may be due in part to the relative youth of our patient cohort, similar to the findings found in other publications on HHD,47 these very positive results require more studies with a larger sample size and longer follow-up time.
Along with the multiple benefits that HHD provides our patients, it is also important to consider the potential drawbacks of the technique, such as psychosocial problems, including the caregiver burden in those patients who are not independent when performing this type of treatment, which can lead to the failure of the technique.48 In this regard, we highlight the low rate of discontinuation of the technique due to caregiver fatigue (n=2; 2.3%) observed in our study. We speculate this low rate could be explained by the simplicity of the dialysis technique and the subjective benefits derived from it, which could make this chronic treatment more bearable for both the patient and their caregiver. In contrast, difficulty in AVF puncture does not seem to be a determining factor in the onset of caregiver fatigue, given that in the two cases described the vascular access was the catheter (data not shown).
In summary, after 12months of follow-up and after analysing multiple parameters, not only analytical, but also in terms of prescription, we have been able to confirm how this system, despite working with a low flow of dialysis fluid, is capable of providing an adequate dialysis dose and is simpler than conventional ones, facilitating acceptance by patients and making it possible for more patients to benefit from this treatment modality. It is necessary to emphasise that this system is designed to perform frequent dialysis, so the beneficial results described in this study and those previously published12 will only be achieved when prescribing frequent dialysis regimens (≥4 days per week).
An important limitation of this study, which is worth emphasising, is its observational nature without a control group, in addition to the relatively small sample size and the short follow-up period. These limitations could lead to a positive selection bias of patients, so the survival analyses should be taken with caution. Although it is the study with the largest number of patients on daily short HHD carried out in Spain, it also provides longitudinal biochemical data of patients treated for the first time with low-flow haemodialysis. It is important to clarify that our results need to be confirmed in prospective studies with a larger sample size and longer follow-up.
HHD with this low dialysate flow system is one more option that is currently available in Spain, but there are other HHD options in terms of machines, filters and types of prescription that also provide multiple benefits,49,50 so we cannot forget that the important thing is to choose the one from among the available options that best suits the needs and circumstances of our patients.
ConclusionsHHD is an unique HD modality in that it offers the opportunity to personalise and intensify dialysis treatment according to the needs and circumstances of each patient, beyond what is normally feasible in a centre-based HD setting. With the results obtained, we can confirm that despite being a system that works with a low flow of dialysis fluid, it has made it possible to obtain good analytical results, maintaining dialysis parameters within the ranges established by current guidelines, with a reduction in medication, a reduction in training time and an adequate survival of the technique throughout the follow-up time. However, more studies are needed to confirm these positive results.
Conflicts of interestMaria Fernanda Slon Roblero: has received speaker and consulting fees from NxStage.
Maria Auxiliadora Bajo Rubio: has received speaker and consulting fees from NxStage.
Mercedes González-Moya: no conflict of interest.
Jesús Calviño Varela: in recent years he has received speaker and consulting fees and fees for attending courses and congresses from Palex, Vifor, Otsuka, Baxter, Astra Zeneca, Alexion, Esteve and Chiesi.
Alejandro Pérez Alba: fees from Baxter for presentations on home haemodialysis.
Juan Villaro Gumpert: no conflict of interest.
Secundino Cigarrán from Hospital da Costa: no conflict of interest.
Pedro Vidau: no conflict of interest.
Sergio Garcia Marcos: no conflict of interest.
Pedro Abáigar Luquin: no conflict of interest.
Elisabet Coll Piera: no conflict of interest.
Antonio Gascón Mariño: no conflict of interest
Maria José Espigares: no conflict of interest.
Mariola D. Molina: no conflict of interest.
Pablo Molina: lecture fees from Abbott, Amgen, Fresenius-Kabi, Nutricia, Palex, Sanofi, and Vifor/Fresenius-Renal Pharma; consulting fees from Fresenius-Kabi, Palex and Vifor/Fresenius-Renal Pharma; and travel grants from Amgen and Fresenius Medical Care.
The authors would like to dedicate the study to all our patients in the HHD programme, true pioneers of this technique in Spain, who teach us so much every day. We also want to thank the nursing staff of all our home dialysis units for their excellent care and educational work. And finally, the authors also thank Manuel Fraile and David Ojeda, specialist nurses in HHD, for sharing their experience and enthusiasm for this type of technique.