Familial hypercholesterolaemia (FH) is one of the most common hereditary metabolic diseases characterised by abnormally high plasma cholesterol levels linked to low density lipoproteins (LDL-C) and a high rate of premature cardiovascular morbidity and mortality.1
FH is associated with mutations in several genes that impairs the ability of the liver to properly remove LDL-C particles from the bloodstream. Although the diagnosis is usually clinical and/or by laboratory tests, genetic testing is necessary for a definitive diagnosis.1,2 It is transmitted in an autosomal dominant manner and the most common cause is genetic mutation of LDL receptors (LDLR); less common are mutations in the apolipoprotein B100 gene (apo-B100) or proprotein convertase subtilisin/kexin type 9 (PCSK9).1
Two monoclonal antibodies which are inhibitors of PCSK9 have recently been approved (evolocumab: Repatha® and alirocumab: Praluent®) for the control of dyslipidaemia in high-risk patients in whom a sufficient reduction of LDL-C is not achieved with the usual lipid-lowering therapies.3
Evolocumab has been authorised for adult patients with primary hypercholesterolaemia (familial heterozygous and non-familial), mixed dyslipidaemia or established atherosclerotic cardiovascular disease, as a complement to the diet: (1) in combination with a statin or with a statin and other lipid-lowering therapies in patients who do not achieve target levels of LDL-C with the maximum tolerated dose of statin; or (2) alone or in combination with other lipid-lowering therapies in patients intolerant to statins or in whom statins are contraindicated; and for patients from 12 years of age with homozygous FH in combination with other lipid-lowering therapies.4
We present the case of a 33-year-old male with a history of obesity, hyperuricaemia and acute myocardial infarction at the age of 25, with angioplasty and stent implantation in the anterior descending coronary artery.
After the cardiac event and the finding of elevated LDL-C (>160mg/dl), treatment was started with rosuvastatin 20mg/day and ezetimibe 10mg/day. Genetic testing located a mutation in the LDLR gene, confirming the diagnosis of heterozygous FH, (mutation M079, genetic identification c.1342C>T, protein identification p.GIn427X).
In 2011, at the age of 27, he was referred to nephrology with proteinuria (0.5g/day) and microhaematuria. After two years of follow-up, due to persistence of proteinuria (0.8g/day), microhaematuria and deterioration in renal function (creatinine 1.42mg/dl), estimated glomerular filtration rate (eGFR) by CKD-EPI 66ml/min/1.73m2, we suggested to perform a renal biopsy, but the patient rejected the idea and discontinued the nephrology follow-up visits.
In 2016, due to poor control of LDL-C he was started on evolocumab (140mg/15 days), and achieved optimal control of his LDL-C levels.
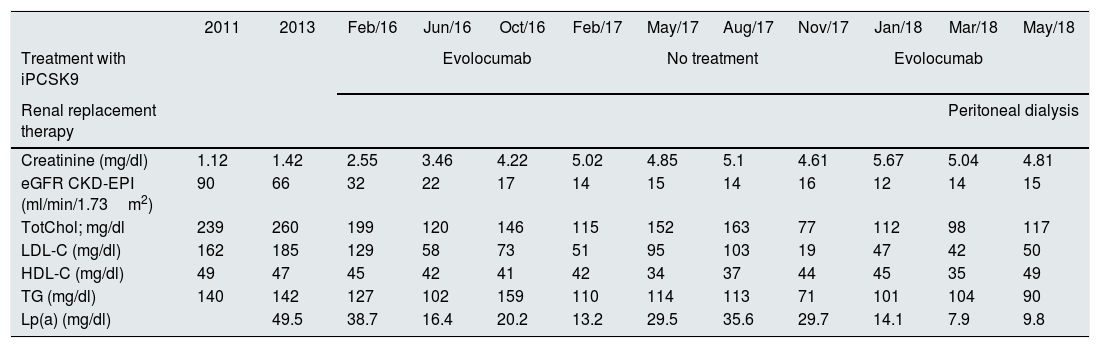
A year later he was referred once again to nephrology with creatinine 4.22mg/dl and eGFR CKD-EPI 17ml/min/1.73m2. Given the rapid progression of the renal failure (RF), although probably due to the natural evolution of his kidney disease, it was decided to withdraw evolocumab after 12 months of treatment because it was not tested in patients with end-stage kidney disease. The patient's LDL-C levels then climbed, despite treatment with rosuvastatin and ezetimibe. In view of the patient's high cardiovascular risk because of the FH and kidney disease, after six months it was decided to restart evolocumab at the same dose, while continuing rosuvastatin and ezetimibe. The patient started the peritoneal dialysis programme in 2018, maintaining adequate lipid control and without evidence of adverse effects attributable to the monoclonal antibody. Table 1 shows the changes in the lipid profile and renal function before and after treatment with evolocumab.
Lipid profile in a patient with heterozygous familial hypercholesterolaemia and chronic kidney disease over the course of the disease according to treatment with evolocumab.
| 2011 | 2013 | Feb/16 | Jun/16 | Oct/16 | Feb/17 | May/17 | Aug/17 | Nov/17 | Jan/18 | Mar/18 | May/18 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment with iPCSK9 | Evolocumab | No treatment | Evolocumab | |||||||||
| Renal replacement therapy | Peritoneal dialysis | |||||||||||
| Creatinine (mg/dl) | 1.12 | 1.42 | 2.55 | 3.46 | 4.22 | 5.02 | 4.85 | 5.1 | 4.61 | 5.67 | 5.04 | 4.81 |
| eGFR CKD-EPI (ml/min/1.73m2) | 90 | 66 | 32 | 22 | 17 | 14 | 15 | 14 | 16 | 12 | 14 | 15 |
| TotChol; mg/dl | 239 | 260 | 199 | 120 | 146 | 115 | 152 | 163 | 77 | 112 | 98 | 117 |
| LDL-C (mg/dl) | 162 | 185 | 129 | 58 | 73 | 51 | 95 | 103 | 19 | 47 | 42 | 50 |
| HDL-C (mg/dl) | 49 | 47 | 45 | 42 | 41 | 42 | 34 | 37 | 44 | 45 | 35 | 49 |
| TG (mg/dl) | 140 | 142 | 127 | 102 | 159 | 110 | 114 | 113 | 71 | 101 | 104 | 90 |
| Lp(a) (mg/dl) | 49.5 | 38.7 | 16.4 | 20.2 | 13.2 | 29.5 | 35.6 | 29.7 | 14.1 | 7.9 | 9.8 | |
eGFR: estimated glomerular filtration rate; HDL-C: HDL cholesterol; iPCSK9: proprotein convertase subtilisin/kexin type 9 inhibitor; LDL-C: LDL cholesterol; Lp(a): lipoprotein(a); TG: triglycerides; TotChol: total cholesterol.
The safety and tolerability of evolocumab was tested for patients with hypercholesterolaemia in the OSLER study.5 However, patients with severe RF (eGFR<30ml/min/1.73m2) have not been studied in phase ii and iii trials.6 No dose adjustment is necessary in patients with mild or moderate RF.7 In phase i the pharmacokinetic and pharmacodynamic results showed that the average evolocumab exposure was lower in patients with severe RF or on haemodialysis compared to subjects without RF.7 The safety data were similar between groups and no clinically significant difference in LDL-C was found.4,7
Despite its high prevalence, FH remains an underdiagnosed and undertreated disease.1 Screening and early diagnosis are necessary for an adequate stratification of cardiovascular risk and effective treatment before the onset of major events.1
There is a real lack of consensus in the clinical guidelines for the management of dyslipidaemia in the renal patient,8 but there are several studies which support the innocuous nature of LDL-C reduction to figures below the current recommendations.9
In our experience, evolocumab has proved to be an effective and safe option for the dislipemia treatment of a patient with FH with advance chronic kidney disease and on peritoneal dialysis.
Please cite this article as: González Sanchidrián S, Labrador Gómez PJ, Aguilar Aguilar JC, Davin Carrero E, Gallego Domínguez S, Gómez-Martino Arroyo JR. Evolocumab para el tratamiento de la hipercolesterolemia familiar heterocigota en enfermedad renal crónica avanzada y diálisis. Nefrologia. 2019;39:218–220.