Restless legs syndrome (RLS) is a neurological disorder characterized by bothersome symptoms associated with impaired quality of life and sleep hygiene. Rotigotine is a novel therapeutic alternative, although few studies have been published in patients on hemodialysis (HD) with RLS treated with rotigotine.
Objectives(1) To establish the prevalence of RLS in our HD unit. (2) To evaluate the efficacy and safety profile of rotigotine and its effect on symptoms, quality of life and sleep hygiene in our HD population with RLS.
Material and methodsA single-center, 12-week prospective study. Two stages (6 weeks): stage 1 (no treatment) and stage 2 (rotigotine). We analyzed: (1) Demographic data, biochemistry data, HD suitability parameters and RLS medical treatment data. (2) Lower extremity symptoms questionnaire (QS). (3) RLS severity symptoms scale (SRLSS). (4) RLS quality of life: John Hopkins RLS-QoL (JH-QoL). (5) Sleep hygiene: SCOPA Scale.
ResultsWe included 66 HD patients, 14 with RLS; 44.4% male, 70.2±9.9 years and 111.1±160.8 months on HD and 22.9% RLS. Exclusively in stage 2, a significant improvement for QS (10±2.4 vs. 5.7±1.0), SRLSS (21±4 vs. 5.7±4.6), JH-QoL (22.1±4.4 vs. 4.3±4.0) and SCOPA (16±5.3 vs. 6.7±1.9) were observed. A 77.7 and 11.1%, showed partial (>20%) and complete (>80%) remission, respectively, while 55.5% achieved “zero” symptoms. Only one patient had gastrointestinal intolerance and none experienced augmentation effect. No changes in biochemical data, suitability for dialysis or medical treatment were found. The inter-group analysis showed a significant improvement in relation to QS, SRLSS, JH-QoL and SCOPA in stage 2.
ConclusionsRLS showed a considerable prevalence in our HD unit. Rotigotine improved clinical symptoms, quality of life and sleep hygiene in RLS patients on HD and was found to be a safe drug with minimal side effects and total therapeutic compliance. Nevertheless, future studies should be performed to confirm the benefits of rotigotine in RLS patients on hemodialysis.
El síndrome de piernas inquietas (SPI) es un trastorno neurológico caracterizado por una molesta sintomatología, asociado a deterioro de calidad de vida e higiene de sueño. Rotigotina constituye una novedosa alternativa terapéutica, si bien existen escasos estudios publicados sobre rotigotina en pacientes en hemodiálisis (HD) con SPI.
Objetivos1.- Establecer la prevalencia de SPI en nuestra unidad de HD. 2.- Evaluar la eficacia y el perfil de seguridad asociado a rotigotina así como su efecto sobre la sintomatología, calidad de vida e higiene del sueño en nuestra población en HD con SPI.
Material y métodosEstudio unicéntrico, prospectivo de 12 semanas de duración. Dos fases (6 semanas): fase 1 (no tratamiento) y fase 2 (rotigotina). Analizamos: 1.- Datos demográficos, bioquímicos, parámetros de adecuación de HD y tratamiento médico relacionado con SPI. 2.- Cuestionario sobre síntomas en extremidades inferiores (QS). 3.- Escala de gravedad de los síntomas (GRLS). 4.- Calidad de vida SPI: John Hopkins RLS-QoL (JH-QoL). 5.- Higiene del sueño: Escala SCOPA.
ResultadosSe incluyó a 66 pacientes en HD. De ellos, 14 con SPI; el 44,4% eran hombres, con 70,2±9,9 años y 111,1±160,8 meses en HD. El 22,9%, con SPI. Únicamente en la fase 2 observamos una mejoría significativa para QS (10±2,4 vs. 5,7±1,0), GRLS (21±4 vs. 5,7±4,6), JH-QoL (22,1±4,4 vs. 4,3±4,0) y SCOPA (16±5,3 vs. 6,7±1,9). Un 77,7 y un 11,1% presentaron remisión parcial (>20%) y completa (>80%), respectivamente. Un 55,5% alcanzó sintomatología «cero». Un único paciente presentó intolerancia digestiva y ninguno, augmentation efect. No observamos cambios en datos bioquímicos, adecuación dialítica ni tratamiento médico. El análisis intergrupos mostró una mejoría significativa en la fase 2 con relación a QS, GRLSS, JH-QoL y SCOPA.
ConclusionesEn nuestro estudio, el SPI urémico presentó una prevalencia considerable. Rotigotina mejoró la sintomatología clínica, la calidad de vida y la higiene de sueño en los pacientes con SPI en HD, por lo que resulta ser un fármaco seguro, con mínimos efectos adversos y con cumplimento terapéutico completo. No obstante, serían necesarios futuros estudios para confirmar el beneficio de rotigotina en la población en HD con SPI.
Restless legs syndrome (RLS) is an entity characterized by an uncontrollable impulse to move limbs, especially lower limbs, accompanied by discomfort or unpleasant sensations in these extremities (such as itching, tingling, pain, stretching, etc.). This occurs after a period of rest and are improved or disappear with movement.1,2
The diagnosis is based on the symptoms observed and the prevalence in the general population is between 5 and 10%; it is more frequent in women and in advanced ages.3,4 Patients with RLS have an impairement in the quality of life, with clinical manifestations of depression or anxiety, as well as a deterioration of sleep habits with daytime sleepiness and fatigue. Some studies even point out a direct relationship between RLS and an increase in mortality.5–7
The alterations of hypothalamic dopaminergic cells in response to elevated levels of some neurotransmitters, the reduction of inhibition at the level of the motor cortex, the hyperreactivity of the spinal flexor reflex or alterations of brain stem reflexes have been some of the diverse and complex neurological mechanisms involved in the pathophysiology of this syndrome.8–10
From the etiological point of view, there are two forms of RLS primary or idiopathic and the secondary. The secondary forms are related to many etiologies, although the most frequent are those associated with anemia with iron deficiency, diabetic neuropathy, neurodegenerative diseases – such as multiple sclerosis or Parkinson's disease – and the presence of advanced chronic kidney disease.11–13
Uremia related RLS is often underdiagnosed. Its prevalence is variable and it is estimated to be between 12 and 30%. The prevalence is higher among patients included in renal replacement therapy programs, mainly in hemodialysis (HD).5,14,15 However, there are not many published studies and they do not include many patients and in some cases with contradictory information.
Currently, the pharmacological therapy of SPI is based mainly on the use of non-ergotamine dopamine agonists.14,15 Its main advantage, compared to the classic use of levodopa and gabapentin, lies mainly in the occurrence of fewer gastrointestinal adverse events. The most commonly used are pramipexole, ropinirole and, more recently, rotigotine.16–18 Rotigotine shows a better control of symptoms, less incidence of both adverse effects and paradoxical potentiation of symptoms (augmentation effect) as well as an ease administration and dosing to the HD patient.18
Given the potential impact of the treatment of RLS on the quality of life and the medical implications, together with the absence of reporto n national series, we thought it would be interesting to conduct this study to describe the prevalence of RLS in our HD unit as well as analyzing the possible effects of rotigotine on the symptoms, quality of life and sleep disorder in our HD patients with RLS.
MethodsDuring the months from January to April 2015, a prospective, 12-week, single-center study was performed in patients with RLS included in regular HD in our center. The study was approved by the Hospital Ethics and Clinical Research Committee and it was carried out in accordance with the Helsinki Declaration.
The inclusion criteria were the following: age equal to or greater than 18 years, with the diagnosis of RLS and signing the informed consent. The exclusion criteria were: lack of informed consent and intellectually unable to answer the questionnaire.
The periodic HD program of our hospital distributes patients in 6 groups of 10–12 patients. These groups perform sessions only in the high-flux HD modality with a 3.5–4h duration on alternate days (L-X-V or M-J-S), during morning, noon (3.5h) and afternoon shifts.
There were two comparative study phases with a duration of six weeks each. In the first phase (phase 1), HD patients at our center were diagnosed of RLS according to the international clinical criteria of the International Restless Leg Syndrome Study Group (IRLSSG, 2012) 1. All patients with RLS were evaluated by the same neurologist. Patients who met 4 criteria were diagnosed of RLS. In the second phase (phase 2), transdermal patches of rotigotine were administered during the first 2 weeks of the week (1mg/day) and in case of correct tolerance and absence of adverse effects, the dose was increased daily up to 2mg/day. After an individualized explanation of the administration system, rotigotine transdermal patches were maintained for 24h, with rotation of the application sites. In case of requiring dose reduction, this was done progressively to avoid a rebound effect on symptomatology.
At the beginning of the study, main demographic data were collected (age, gender, ethiology of renal failure, vintage) and associated comorbidities (diabetes mellitus, arterial hypertension, peripheral vascular disease, ischemic heart disease, Charlson comorbidity index, smoking, alcohol habit, caffeine derivatives). Both at the beginning and at the end of each of phases, the main biochemical data were analyzed, the patient's usual medication related to RLS, as well as the characteristics and adequacy data in HD (Kt/V, method 2nd generation Daugirdas). Also, the following variables were analyzed.
Degree of severity of restless legs syndrome symptoms and questionnaire on specific symptomsThe degree of severity of the symptoms was determined by the severity scale (GRLS) established by the IRLSSG19 and the symptomatology in both lower limbs by means of a questionnaire of specific symptoms (QS). The GRLS is a validated survey in relation to the repercussion of the SPI symptomatology in each patient. It consists of 10 questions with 5 possible answers. Each answer is scored from 0 to 4. The simplest way to analyze the result is the sum of the total score. From 15 points up, it is considered severe symptomatology. The higher the score, the greater the severity. The QS assesses using a qualitative scale (1: null, 2–3: little 4: enough, 5: a lot) the presence of the following symptoms: muscle pain, cramps, tingling, burning and numbness.
Quality of life and sleep habitsQuality of life was assessed using an approved health questionnaire adapted to RLS: John Hopkins RLS QoL (JH-QoL).20 Includes 18 questions with 5 possible answers and a score from 0 to 4. The simplest way to analyze the results is the sum of the total score. Overall, the higher the score, the poorer quality of life for a RLS patient. The sleep habit was evaluated using the validated SCOPA21 survey. It consists of 2 sections; the first evaluates the nighttime sleep. It consists of 6 questions, 5 more specific and valued from 0 (not) to 3 (much) and a sixth question that is dedicated to global assessment with 7 possibilities from 0 (very bad) to 7 (very good). In the second section daytime sleepiness is assessed. It consists of 6 specific questions valued from 0 (never) to 3 (frequently). The global analysis is done by the sum of points in each section (nocturnal sleep/daytime sleepiness).
Efficacy of the treatment, therapeutic completion and adverse effectsWith regard to the effectiveness of the treatment, a partial response was defined as a reduction of more than 20% in the QS. A complete response was defined as a reduction of more than 80% in the QS and the presence of “zero” symptoms was defined by a score lower than 5 points in the QS.
Compliance with the treatment was evaluated by reviewing the transdermal patch at the beginning of the HD session. Those patients who presented absence of the transdermal patch in 3 regular revisions were classified as noncompliant.
Adverse effects analyzed by anamnesis during the usual sessions of HD were: allergic-type reactions in the area of application (erythema or induration, local pain, pruritus), headache, drowsiness, nausea, vomiting, dyspepsia, hypertension and paradoxical potentiation of the symptoms (augmentation effect).
The statistical analysis was performed using the SPSS program version 18.0 (SPSS Inc, Chicago, IL, USA). By Kolmogorov–Smirnov test the data did not present a normal distribution. The quantitative variables were expressed by means and standard deviation. The qualitative variables, by percentage. The quantitative data were compared using the Wilcoxon test for nonparametric related variables and the qualitative data using the McNemar test. The intergroup analysis was carried out using the Mann–Whitney U test, considering a p<0.05 value as statistical significance.
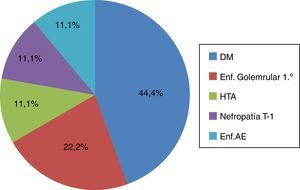
ResultsA total of 66 patients prevalent in the HD program were analyzed. Five were excluded (3 had dementia, one did not accept to participate and another patient had a psychiatric disorder). Of the remaining 61 patients, 14 were diagnosed with RLS, with a prevalence of 22.9%. During the study period there were 5 dropouts (one due to cardiorespiratory arrest, 3 due to revocation of informed consent and one due to malignant hematological disease), none of dropouts was the related to the present study. Of the 9 remaining patients, 44.4% were men, with an average age of 70.2 years±9.9 years and a mean time on HD of 111.1±160.8 months. The average Charlson index was 10.2±2. The main comorbidities were: arterial hypertension (88.9%), diabetes mellitus (77.8%), peripheral vascular disease (55.6%) and ischemic heart disease (22.2%). The main etiology of chronic kidney disease was diabetes mellitus (44.4%). The rest of the etiologies are shown in Fig. 1. Among the potential triggers, 44.4% consumed caffeine derivatives on a regular basis, 25% had alcohol consumption and 35.7% had active smoking.
Causes of chronic kidney disease in patients with RLS in HD. Results expressed in percentages (%). DM: diabetes mellitus; AE Dis.: atheroembolic disease; primary glomerular Dis: primary glomerular disease; HTN: hypertension; TI nephropathy: tubulointerstitial nephropathy; RLS: restless legs syndrome.
No significant differences were observed in the main biochemical parameters and HD adequacy in both phases of the study (Table 1). Similarly, no changes were made in the patient's usual medication related to RLS during the study (88.9% conventional analgesia, 33.3% antidepressants or anxiolytics and 11.1% took opioids, respectively). No patient was taking levodopa, clonidine, gabapentin, l-carnitine or antiemetics. The characteristics of the HD filter were maintained (helixone 33%, polysulfone 44%, polysulfone hi-flow 11%, polyacrylonitrile 11%) and no relevant changes were observed in the usual treatment given during HD sessions throughout the study (iron intravenous 77.8 vs. 72.5%, erythropoietic agents 30.9±11.6 vs. 28.8±10.4mcg darbopoetin/week).
Biochemical parameters and adequacy of hemodialysis.
| Phase 1 | Phase 2 | |||
|---|---|---|---|---|
| Initial | Final | Initial | Final | |
| Biochemical data | ||||
| Urea, mg/dl | 237.2 (80.6) | 234.7 (85.4) | 236.6 (85.2) | 238.1 (87.6) |
| Glucose, mg/dl | 153.2 (51.6) | 155.8 (62.4) | 152.2 (32.1) | 155.4 (36.1) |
| Creatinine, mg/dl | 8.1 (2.3) | 8.2 (3.1) | 8.2 (2.3) | 8.1 (2.6) |
| K, mEq/L | 5.2 (0.8) | 5.5 (1.1) | 5.1 (0.2) | 5.2 (0.7) |
| Ca, mg/dl | 9.2 (0.4) | 9.2 (0.8) | 9.2 (0.4) | 9.3 (0.8) |
| P, mg/dl | 4.4 (1.2) | 4.3 (1.2) | 4.4 (1.2) | 4.3 (1.1) |
| Mg, mg/dl | 1.9 (0.9) | 1.9 (0.8) | 1.8 (0.7) | 1.9 (0.8) |
| i-PTH, pg/ml | 257.6 (189.6) | 265.8 (196.2) | 264.4 (110.7) | 258.4 (183.2) |
| 25-OH vitD, ng/ml | 23.3 (8.1) | 26.7 (11.6) | 28.2 (13.5) | 27.1 (7.2) |
| Albumin, g/dL | 3.7 (0.3) | 3.7 (0.2) | 3.8 (0.2) | 3.7 (0.2) |
| Prealbumin, mg/dl | 28.5 (10.5) | 28.1 (4.8) | 29.2 (6.7) | 29.7 (7.2) |
| Total cholesterol, mg/dl | 143.2 (57.2) | 148.1 (59.1) | 153.2 (34.4) | 149.9 (34.8) |
| HDL cholesterol, mg/dl | 42.6 (11.1) | 41.2 (11.9) | 42.7 (10.3) | 41.2 (43.6) |
| LDL cholesterol, mg/dl | 68.7 (42.3) | 63.4 (49.2) | 67.2 (65.4) | 65.9 (33.7) |
| Triglycerides, mg/dl | 156.1 (74.1) | 158.6 (72.6) | 157.2 (70.6) | 157.2 (71.2) |
| Hemogram | ||||
| Hemoglobin, g/dl | 10.8 (1.8) | 11.1 (0.9) | 10.5 (1.2) | 10.9 (0.7) |
| Ferritin, ng/ml | 411.6 (210.7) | 427.2 (267.8) | 424.1 (204.9) | 421.9 (300.1) |
| Hs-CRP, ng/L | 7.8 (1.5) | 8.1 (1.7) | 8.1 (1.8) | 8.3 (1.2) |
| Dialysis adequacy | ||||
| Weekly hours of HD | 11.1 (0.8) | 11.1 (0.8) | 11.1 (0.8) | 11.1 (0.8) |
| Dose of dialysis, Kt/V | 1.62 (0.4) | 1.63 (0.7) | 1.63 (0.5) | 1.62 (0.6) |
Phase 1 and phase 2 (rotigotine 2mg/day): results are expressed as mean (standard deviation), initial vs. final.
Ca: calcium; Hs-CRP: C-reactive protein; i-PTH: intact parathyroid hormone; K: potassium; Kt/V: 2nd generation method Daurgirdas; Mg: magnesium; P: phosphorus; VitD: vitamin D.
No significant differences were found between the groups studied.
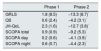
The values of the main variables analyzed in the different phases of the study are shown in Table 2. Regarding the severity of the symptoms, 73.3% had severe symptoms (GRLS>P15), 35.7% reported cramping, 28.5% numbness and 21.4% muscle pain and burning, in the QS of the lower extremities. No relevant changes were observed in the GRLS scale (19.1±9.2 vs. 21.1±4.2, p<0.524) or in the QS questionnaire (9.3±1.7 vs 10.2±2.4; p<0.428) during phase 1 of the study.
Scale for severity of symptom (GRLS), lower extremity symptoms questionnaire (QS), quality of life related to RLS (JH-QoL) and sleep habits (SCOPA).
| Phase 1 | Phase 2 | |||
|---|---|---|---|---|
| Initial | Final | Initial | Final | |
| GRLS | 19.1 (9.2) | 21.1 (4.2) | 21.1 (4.2) | 5.7 (4.6)* |
| QS | 9.3 (1.7) | 10.2 (2.4) | 10.2 (2.4) | 5.7 (1.1)* |
| JH-QoL | 19.8 (9.2) | 22.1 (4.4) | 22.1 (4.4) | 4.3 (4.1)* |
| SCOPA total | 15.1 (5.3) | 16 (5.3) | 16 (5.3) | 6.7 (1.9)* |
| SCOPA day | 5.3 (3.6) | 5.6 (3.6) | 5.6 (3.6) | 1.4 (0.7)* |
| SCOPA night | 9.8 (2.7) | 10.4 (2.7) | 10.4 (2.7) | 6.1 (1.1)* |
Phase 1 and phase 2 (rotigotine 2mg/day): Results expressed as mean (standard deviation), initial vs. final.
GRLS: scale of severity of symptoms of the International Restless Leg Syndrome Study Group; JH-QoL: John Hopkins restless leg syndrome quality of life; QS: scale of symptoms of lower extremities; SCOPA: sleep habits scale.
During phase 2 of the study, we observed a significant improvement in GRLS (21.1±43 vs. 5.7±4.6; p<0.001) and QS (10.2±2.4 vs. 5.7±1.1; p>0.004) after the introduction of rotigotine treatment. The dose of transdermal rotigotine administered was 2mg/day, with complete therapeutic compliance. A 88.8% of the patients responded to the treatment with rotigotine, with an average reduction of 15.2±17.2 points in the GRLS scale at the end of phase 2 of the study. A 77.7% presented a partial response (reduction greater than 20% QS) and 11.1% presented a complete remission (reduction greater than 80% in QS). A 55.5% reached “zero” symptoms, defined by a score lower than 5 points in the QS. No allergic reactions were observed in the application area. Only one patient presented mild gastro intestinal intolerance in the form of dyspepsia, which yielded after the reduction of rotigotine to 1mg/day. None of the patients in our study presented augmentation effect.
As far as quality of life related to RLS and sleep habits, we observed a worsening for the JH-QoL scales (19.8±43 vs. 22.1±4.4; p<0.096) and SCOPA (total 15.1±5.3 vs. 16±5.3, p<0.124; diurnal, 5.3±3.6 vs. 5.5±3.6, p<0.368; nocturnal 9.8±2.7 vs. 10.4±2.7; p 0.347) during phase 1 of the study, although these results did not reach statistical significance. Overall, after the introduction of rotigotine (phase 2), we observed a significant improvement in terms of quality of life related to SPI (JH-QoL 22.1±4.4 vs. 4.3±4.1; p<0.004) and in the different sections of sleep habits (total SCOPA 16±5.3 vs. 6.7±1.9; p<0.001; diurnal 5.5±3.6 vs. 1.4±0.7; p<0.013; nocturnal 10.4±2.7 vs. 6.1±1.1; p 0.001).
Table 3 shows the difference of means of the main variables in the different phases of the study. There was a significant improvement in terms of severity of symptoms (1.8±8.5 vs. −13.3±8.7; p<0.003), presence of specific symptoms in the lower extremities (0.6±2.4 vs. −4.2±3.1; p<0.004), quality of life related to RLS (2.3±1.6 vs. −12.7±3.8; p<0.001) and sleep habits (total 0.9±1.1 vs. −9.2±5.3; p<0.001; diurnal 0.2±0.6 vs 4.1±3.9, p<0.001, nocturnal 0.6±0.7 vs. −4.4±2.3, p<0.001), after the introduction of rotigotine.
Analysis of differences between study groups.
| Phase 1 | Phase 2 | |
|---|---|---|
| GRLS | 1.8 (8.5) | −13.3 (8.7)* |
| QS | 0.6 (2.4) | −4.2 (3.1)* |
| JH-QoL | 2.3 (1.6) | −12.7 (3.8)* |
| SCOPA total | 0.9 (0.9) | −9.2 (5.3)* |
| SCOPA day | 0.2 (0.6) | −4.1 (3.9)* |
| SCOPA night | 0.6 (0.7) | −4.4 (2.3)* |
Analysis of the mean changes of the main variables between the study groups. Results expressed as mean (standard deviation).
GRLS: scale of severity of symptoms of the International Restless Leg Syndrome Study Group; JH-QoL: John Hopkins restless leg syndrome quality of life; QS: scale of symptoms of lower extremities; SCOPA: sleep habits scale.
Analysis using the nonparametric statistical test U of Mann–Whitney.
The results of our study show that the RLS is frequent in our HD unit. In these patients, the use of rotigotine improved the clinical symptoms, quality of life and sleep habits; it is a safe drug, with minimal adverse effects and complete therapeutic compliance.
The prevalence of RLS in our HD unit, it is practically the same as previously described in the literature4,22: it ranges from 12 and 30%, despite the high comorbidity and prolonged time on HD. Patients with RLS have a decreased in life quality with depression and anxiety as well as a abnormalities in sleep habits producing daytime sleepiness and fatigue.5,19,20 These data are similar to those observed in our study, in which more than 75.5% of patients had severe symptoms. The presence of all these symptoms affected quality of life and sleep habits.
Currently, therapy with non-ergotamine dopamine agonists has become the first-line of therapy for moderate-severe involvement of RLS. The most commonly used medications are pramipexole, ropinirole and, more recently, rotigotine.15–17 However, there are still few published work evaluating the best treatment of RLS in HD patients23 (Table 4).
Main randomized clinical trials with pharmacological intervention in patients with restless legs syndrome in renal replacement therapy.
| Author (country, year) | n | Intervention groups | Weeks | Variables being analyzed | Main results |
|---|---|---|---|---|---|
| Trenkwalder (Germany, 1995) | 28 (11 HD) | Intervention: madopar (L-dopa 100mg+benserazide 25mg) Control: placebo | 4 | Own questionnaire on severity of symptoms RLS, PLM, LQ (Hamburger AVS), CGI, AE | L-dopa significantly improved the main variables analyzed, without relevant adverse effects |
| Thorp (USA, 2001) | 16 | Intervention: gabapentin (200–300mg/post-HD day) Control: placebo | 6 | Own questionnaire on severity of symptoms, AE | 2 patients abandoned due to lethargy attributed to gabapentin Gabapentin was an effective drug in the treatment of RLS in HD patients |
| Sloand (USA, 2004) | 25 | Intervention: Fe dextran iv (1.000mg) Control: normal saline iv | 4 | Severity of symptoms SPI (Rochester), biochemical data Iron kinetics, AE | Headache, nausea and vomiting, without differences between groups Iron dextran was associated with improvement of RLS symptoms and elevation of iron levels |
| Micozkadioglu (Turkey, 2004) | 15 | Intervention: gabapentin (200mg/post-HD day) Control: levodopa 125mg/day | 4 | RLS-6, CV (SF-36), Sleep quality (PSQI), AE | One patient left due to fatigue and syncope in gabapentin group Significant reduction in gabapentin group: RLS-6, SF-36 and PSQI |
| Pellecchia (Italy, 2004) | 11 | Intervention: ropinirol (0.25–2mg/day) Control: levodopa continuous release (25/100–50/200mg/day) | 6 | GRLS, sleep period (polysomnography), AE | Ropinirol was superior to continuous release of levodopa in the treatment of RLS, without relevant AE |
| Sagheb (Iran, 2012) | 60 | Intervention: 4 groups of 15 patients: vitamin C (200mg) and vitamin E (400mg); vitamin C (200mg) and placebo; vitamin E (400mg) and placebo; double blind | 8 | GRLS, AE | Short term improvement of GRLS with Vitamin C and E as well as their combination. |
| Dauviliers (USA, 2016) | 30 | Intervention rotigotine (1–3mg/day) Control: placebo | 2 | PLM, GRLS, CGI | Nausea and dyspepsia in vitamin groups |
CGI: clinical global impression; LQ: quality of life; AE: adverse effects; USA UU: United States; GRLS: symptom severity scale of the International Restless Leg Syndrome Study Group; HD: hemodialysis; iv: intravenous; PLM: periodic leg movements; PSQI: Pittsburgh Sleep Quality Index; RLS-6: Restless Leg Syndrome scale 6; RLS: restless legs syndrome; AVS: analog visual scale.
Within this group of drugs, the use of rotigotine has been extended in the every day practice due to the efficacy, good tolerance, few adverse effects, low prevalence of paradoxical potentiation of symptoms or augmentation effect and no need for dose adjustment in CKD patients.24,25 These advantages are mainly due to the administration in the form of transdermal patch, which guarantees a continuous delivery throughout the day achieving stable serum levels that minimize the possibility of accumulation and there is no need to monitor blood levels and adverse effects are uncommon. In addition transdermal application facilitates therapeutic adherence, by decreasing the number of tablets the majority of patients on multiple medications, facilitating therapeutic compliance. The draw back is the high cost, superior to the rest of the non-ergotamine agonists. In our study, rotigotine was a safe and effective drug, with minimal adverse effects and complete therapeutic compliance, there were no dropouts and achieved a significant improvement in clinical symptoms, quality of life related to RLS and sleep habits.
To date, uremic RLS has been underestimated as evidenced by the fact that none of the patients diagnosed received specific treatment for RLS. Among other reasons, this was probably due to: the fact that symptoms in the lower limbs could be attributed to other factors or associated comorbidities, the scant interest by the medical staff due in part to the lack of physiopathological knowledge of this entity and the absence of an effective treatment free of relevant adverse effects. All these aspects have lead to the use of various medications without clear guidelines, based on the clinical experiences themselves or on a few published works.
There is a significant prevalence of RLS, a pathology that causes significant symptomatology associated to deterioration of the quality of life, without lack of specific abnormalities in blood count or biochemical parameters. Based on the results obtained in this study we should consider establishing different strategies to assess the presence of RLS in our patients.
In this sense, all the incident patients in our HD unit will be evaluated for RLS according to the clinical criteria of the IRLSSG. Those cases with reasonable doubts will be discussed with the Neurology Service for a more exhaustive assessment. In the same way, the severity of the symptoms and the quality of life and sleep habits will be assessed by means of the tests described above and, in addition the patient will receive dietetic advice and the appropriate medical treatment according to the individual clinical characteristics.
Within the multiple limitations of our study, we must mention the small number of patients included which forced the use of nonparametric statistical tests, as well as the short time of follow-up. The sample size was conditioned by our limited population in HD, while the 12-week period was established to avoid possible confounding effect derived from the usual changes in medications after the quarterly analytical determinations in our HD unit. Therefore, we cannot rule out the possibility that a longer period of followup could entail some change in relation to the effectiveness, safety and tolerance of rotigotine. Studies with a larger number of patients and longer duration would be necessary to confirm the benefits of rotigotine observed in our patients in HD.
In conclusion, we can state that there is considerable prevalence RLS, with an intense symptomatology and a deteriorated quality of life and sleep habits. The use of rotigotine improved clinical symptoms, quality of life and sleep habits in HD patients with RLS. It proved to be a safe drug, with minimal adverse effects and complete therapeutic compliance. However, future studies would be necessary to confirm the benefit of rotigotine in the HD population with RLS.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Esteve V, Carneiro J, Salazar G, Pou M, Tapia I, Fulquet M, et al. Efectos de la rotigotina sobre la sintomatología, calidad de vida e higiene de sueño en el síndrome de piernas inquietas en hemodiálisis. Nefrologia. 2018;38:79–86.