Background: Tuberous sclerosis (TS) is a systemic disease, with an autosomal dominant pattern of inheritance caused by mutations in two genes (TSC1 and TSC2) that cause tumours (angiomyolipomas [AML], angiofibromas, astrocytomas). Constant and inadequate proliferation occurring in TS may be blocked by mTOR inhibitors (mammalian target of rapamycin), such as rapamycin. Material and methods: At present, our study includes 17 patients with TS. All had at least one AML greater than 2cm in diameter diagnosed by MRI. They received rapamycin during 12 months. Plasma levels remained stable between 4-8ng/dl. The AML size was monitored every six months by abdominal MRI. Results: At 12 months of inclusion, MRI indicated a decrease in the size of AML in all patients showing at least a 50% reduction in 82.4% (14/17, 95% CI [56.57%, 96.20%]). The mean percent reduction was 66.3% (95% CI [56.9%, 75.6%], P<.0001). The major side effects observed were: oral aphthous ulcers (5/17); hypertriglyceridemia (3/17); microcytosis and hypochromia (3/17); diarrhea (2/17); acne (1/17); acute pyelonephritis (1/17); and proteinuria (1/17). Conclusions: These preliminary clinical data suggest that rapamycin can play a beneficial role in the treatment of TS. Our experience in 17 patients treated for 12 months demonstrates safety and efficacy in reducing AML volume.
Introducción: La esclerosis tuberosa (ET) es una enfermedad sistémica, de herencia autosómica dominante, ocasionada por mutaciones en dos genes (TSC1 y TSC2), que causan la aparición de tumores (angiolipomas [AML], angiofibromas, astrocitomas, etc.). La proliferación inadecuada y constante que existe en la ET puede ser bloqueada por inhibidores de la kinasa mTOR (mammalian target of rapamycin), como la rapamicina. Material y métodos: Se han incluido 17 pacientes afectados de ET y, al menos, un AML mayor de 2 cm de diámetro diagnosticado por resonancia magnética (RM). Han recibido tratamiento con rapamicina durante 12 meses. Los niveles plásmáticos se han mantenido entre 4 y 8 ng/dl. El tamaño del AML se ha monitorizado semestralmente mediante RM abdominal. Resultados: A los 12 meses de la inclusión, con la RM se ha objetivado una disminución del tamaño del AML en todos los pacientes incluidos, mostrando una reducción de, al menos, un 50% en el 82,4% (14/17; intervalo de confianza [IC] 95% [56,57%, 96,20%]). El porcentaje medio de reducción fue del 66,3% (IC95 [56,9%, 75,6%]; p <0,0001). Los principales efectos secundarios observados han sido: aftas orales (5/17); hipertrigliceridemia (3/17); microcitosis e hipocromía (3/17); diarrea (2/17); acné (1/17); pielonefritis aguda (1/17), y proteinuria (1/17). Conclusiones: Los datos clínicos preliminares sugieren que la rapamicina puede desempeñar un papel beneficioso en el tratamiento de la ET. Nuestra experiencia en 17 pacientes tratados durante 12 meses demuestra seguridad y eficacia en la reducción de AML.
INTRODUCTION
Tuberous sclerosis (TS), otherwise known as Bourneville-Pringle disease, is a systemic disease with an autosomal dominant pattern of inheritance. It is a rare disease, with an estimated prevalence of 1/6000 people.1
This disease can cause dermatological (facial angiofibromas, hypomelanotic macules, ungual fibromas), renal (angiomyolipomas, cysts) neurological (epilepsy, mental retardation, subependymal astrocytomas, cortical tubers) pulmonary (lymphangioleiomyomatosis), and cardiac (rhabdomyomas) symptoms. These clinical manifestations and their severity vary greatly. The clinical presentation of the disease varies from adults with very few signs of the disease to children with severe neurological involvement.
Renal angiomyolipomas (AML) are benign tumours composed of anomalous vessels, immature smooth muscle tissue, and adipocytes. They can be detected using ultrasound, computerised tomography (CT), and magnetic resonance (MRI). In the majority of patients, they occur as bilateral and multiple tumours. The approximate incidence ranges between 55% and 75%.2 and morbidity rates are high. They can produce spontaneous haemorrhage and, in some cases of abundant AML, arterial hypertension (AHT) and kidney failure. This is the most serious non-neurological complication of the disease.
The risk of rupture and spontaneous haemorrhage is generally related to the size of the AML3 and is especially high when the tumour is larger than 3-4cm. The growth rate of AML varies among patients and tumours. In general, resection is avoided in order to prevent the loss of renal parenchyma, and thus renal function. Until now, the primary therapeutic options have been AML embolisation, elective surgery, and emergency nephrectomy in the case of uncontrollable haemorrhage.4
In addition to renal AML, patients with TS may have cysts, polycystic kidney disease, and renal cell carcinomas.
TS is caused by mutations in two different genes: TSC1 and TSC2. TSC1 causes the disease in a small percentage of cases and produces its most benign forms.2 This gene is located on chromosome 9q34, is made up of 23 exons, and codes for the protein hamartin.5 TSC2 is located on chromosome 16p13, is made up of 41 exons, and codes for the protein tuberin.6
Tuberin and hamartin join in a regulatory complex of mTOR (mammalian target of rapamycin) through the ribosomal protein S6 kinase (S6K) and the repressors of the protein synthesis initiation factor eIF4EEl, the 4E binding protein (4EBP1). Mutations that result in the absence or dysfunction of tuberin or hamartin cause a constitutive inactivation of S6K and 4E-BP1, and a loss of control of cell proliferation7 caused by permanent activation of mTOR.
Rapamycin (Sirolimus, Rapamune®) is an immunosuppressive agent that inhibits the capacity of mTOR to phosphorylate S6K and 4EBP1, which controls cell growth and the uninhibited proliferation produced in TS patients.
The objective of this clinical trial is to demonstrate that the uninhibited proliferation that exists in TS patients – AML – can be blocked by inhibitors of the Akt signalling cascade, such as rapamycin, which is a safe and effective therapeutic alternative in the treatment of TS patients.
MATERIAL AND METHOD
Ours is a phase IV non-blinded, non-controlled clinical trial, lasting 24 months. It is being held at a single institution, using a commercialised drug under a new therapeutic indication.
Our primary objective is to evaluate the effect of rapamycin on the size of AML in patients with TS. The main variable is the estimated proportion of patients in which a 50% reduction was observed in the largest diameter of the AML with respect to its initial size.
Our secondary objectives are to evaluate the treatment effect on tumour volume, cutaneous lesions, the percentage of patients with surgical complications (haemorrhage, need for embolisation and/or surgery), and the safety of the drug in this cohort of patients.
The majority of patients included in our study belong to the TS association, which referred possible candidates to the Puigvert Foundation, which is responsible for this study.
This clinical trial has been approved by the clinical research ethics committee of the Puigvert Foundation and the Agencia Española del Medicamento (Spanish agency of drugs).
We included 17 patients older than 10 years of age, diagnosed with TS and with at least one renal AML greater than 2cm in diameter (independently of the central nervous system [CNS], heart, lungs, and/or skin involvement), and baseline creatinine levels below 2mg/dl. All patients were also required to sign an informed consent if they were older than 18 years, or this was signed by parents or guardians if the patient was underage. We established the following exclusion criteria: recent haemorrhage of the AML, altered liver function or haemogram results, proteinuria (estimated using the protein/creatinine ratio) greater than 22.6mg/mmol, active infection, a background of surgery within 8 weeks before to the start of treatment, history of neoplasia within the past 2 years, a fasting cholesterol level greater than 7.8mmol/L, LDL greater than 5.1mmol/L, triglycerides (TG) greater than 4.6mmol/L, and a background of allergies to macrolides. We also performed a pregnancy test on all female patients before including them in the study, and continued to test for pregnancy during each follow-up visit.
Each patient received a dose of rapamycin at 1mg/day in the first visit. Dosage was adjusted every two weeks, with 1mg increases until reaching stable plasma levels of 4-8ng/dl. Once these levels were reached, we monitored plasma levels at 3, 6, 9, and 12 months of treatment, coinciding with the follow-up visits. During each visit the patients underwent a physical examination, adverse effects and compliance with the therapeutic protocol were evaluated, and lesions were photographed. They also underwent a complete blood analysis (glucose, haemogram, urea, creatinine, MDRD formula [Modification of Diet in Renal Disease], electrolytes, liver profile, bilirubin, lipids, and urine analysis for proteinuria using the protein/creatinine ratio). We performed an abdominal MRI on each patient upon inclusion in the study and at 6 and 12 months.
Apart from the follow-up visits, each patient was also contacted on a monthly basis by telephone. Patients also had direct telephone access to the research team for any incidents during the research period.
We registered adverse effects by evaluating the description, duration, and treatment of each incident, and the researcher also determined what caused the patient’s condition. Severe and unexpected adverse reactions were reported to health care authorities and the clinical research ethics committee in accordance with the stipulations of the Royal Decree/2004.
We predetermined a sample size of 17 patients to obtain at least a statistical power of 80% in order to detect differences between the study group and the expected efficacy in the general population with no treatment, which is 0%, with a two-tailed 5% type I error.8,9
Given that our study design did not include a control group, we were not able to test additional hypotheses, although we did estimate 95% confidence intervals (CI) for the primary variable using exact methods, and we have discussed our results in the context of previously observed values for this pathology in the general population.
The tumour volume was analysed using a restricted maximum likelihood model for repeated measures with an unstructured covariance matrix. We introduced the baseline value as a covariable for the models designed for testing difference and percent differences with regard to initial values. We established 5% as the two-tailed significance level, and all data were analysed using SAS software, version 9.1.3 (SAS Institute Inc., Cary, NC, USA).
Study limitations
Design limitations
We have undertaken a study with no controls and a reduced number of patients, which implies limitations in methodology and interpretation of results. This study design was due to the low prevalence of the disease and the limited availability of patients in Spain; however, we do consider it to be of great interest to be able to perform a clinical trial for such a rare disease in which no drug treatment exists, and which is associated with a high rate of morbidity. Our study has been carried out in a single institution in order to maximise the consistency of criteria for assessing the patient response.
RESULTS
Sixteen out of the 17 patients included in the study (8 men and 9 women) completed the treatment for the full 12 months. One had to be withdrawn from the study after 11 months due to reactivation of an erythema nodosum which was already present at the start of the trial, and another patient was removed from the study after 13 months of treatment due to the appearance of nephrotic proteinuria which disappeared after treatment suspension.
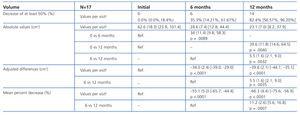
The sizes of the AML at the start of the study and after 6 and 12 months of treatment are shown in Table 1, along with the mean percent decrease in AML volume at 6 and 12 months. After 6 months, 35.3% (6/17, 95% CI [14.21%, 61.67%]) of tumours had decreased in size by 50%, and 82.4% (14/17, 95% CI [56.57%, 96.20%]) after 12 months. The mean percent decrease in volume after 6 months was 55.1% (95% CI [44.4%, 65.7%]; P<.0001), and was 66.3% after 12 months (95% CI [56.9%, 75.6%]; P<.0001). Figures 1 and 2 show the evolution of the lesion volume, which was analysed in terms of absolute decrease in volume and mean percent volume decrease.
With treatment, facial angiofibromas decreased in size, and became lighter and smoother.
We observed no significant differences in creatinine levels between initial values and values after 12 months of treatment, although microalbuminuria did appear, but did not exceed 300mg/24 hours, except for one patient that developed nephrotic proteinuria which was resolved by halting treatment.
The most frequently observed adverse effects (Table 2) were the appearance of oral aphthous ulcers at the start of treatment (resolved using topical corticosteroids), hypertriglyceridemia (controlled using medical treatment), self-limited episodes of diarrhoea, acne and microcytosis, and hypochromia related to the antiproliferative effect of rapamycin. One patient required hospitalisation due to acute pyelonephritis.
DISCUSSION
Currently, given the absence of treatment options for this rare disease, we must expand our knowledge of the biological, cellular, and molecular aspects of TS, and research effective therapeutic alternatives.
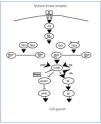
In this vein, studies performed with Drosophila have demonstrated that the loss of tuberin produces a defect in the cell cycle that causes the cell to repeat S phase without entering M phase.10 In this study, as in others, tuberin and hamartin were found to be key components of the PI3K/PKB(Akt)/mTOR/S6K signalling cascade, which regulates nutrient uptake, cell size, and cell proliferation (Figure 3).11-13
Tuberin and hamartin join in a complex that functions as the most important regulator of the mTOR kinase in the Akt cascade through an intermediate protein called Rheb.13,14 It appears that mTOR mediates the majority of its effects on cell growth through phosphorylation by way of the ribosomal protein S6 kinase (S6K) and the repressors of the protein synthesis initiation factor eIF4EEl, the 4EBP1. S6K increases cell growth and protein synthesis, whereas 4EBP1 inhibits these processes. mTOR interacts with S6K and 4EBP1 through an associated protein: Raptor. The intact tuberin/hamartin complex keeps Rheb in an inactive, dephosphorylated state (Rheb DGP). The phosphorylation of tuberin by Akt inactivates the GAP activity of the tuberin/hamartin complex and provokes its dissociation, which leads to elevated levels of Rheb-GTP and allows for the activation of specific targets in the later steps of the mTOR-S6 cascade and the initiation of the 4E-BP1 inhibition factor.
Mutations that cause the absence or dysfunction of tuberin or hamartin, such as those that occur in TS patients, produce a constitutive inactivation of S6K and 4E-BP1 as well as a loss of control of cell proliferation.7
Rapamycin (Sirolimus, Rapamune®) is an immunosuppressive agent that forms an inhibitory complex with the immunophilin (FKBP12), which joins to the FK-506 binding protein 12 (FKBP-12) and inhibits the capacity of mTOR to phosphorylate S6K and 4EBP, which, in turn, inhibits the proliferation of T-cells. This drug is on the market, with indications for prophylaxis of acute kidney graft rejection in adults with low immunological risk. This drug is administered orally, and is absorbed poorly but quickly, with an oral bioavailability of 15%. Maximum plasma concentrations are reached within 0.5-2.3 hours. Roughly 95% of the medication is taken up by red blood cells and is distributed throughout the organism, even passing through the blood–brain barrier. It is metabolised by the liver CYP3A4, with a mean lifetime of 57-62 hours.
The hypothesis of this trial is that the uninhibited proliferation observed in TS in the form of AML and astrocytomas could be blocked by inhibitors of the Akt signalling cascade, such as rapamycin. This drug has been demonstrated to reverse the cell size defect in flies with TS and reduces neoplastic growth in rats and mice with TS.15 This drug also reduces vascular endothelial growth factor (VEGF) levels, and taking into consideration the high vascularisation of TS tumours caused by upregulation of VEGF, its antiangiogenic activity could be very beneficial.
Preliminary clinical data suggest that rapamycin could play a beneficial role in the treatment of TS. The currently available medical literature on the subject is limited to publications on isolated cases of reduced size of astrocytomas and AML in patients with TS with good tolerance,16-19 and the results from one clinical trial.20 Several different clinical trials are currently underway. One phase II trial with no controls demonstrated a decrease in renal AML size (49.92%±15.62%) and improved functional respiratory test results (forced expiratory volume in one second [FEV1], forced vital capacity, and residual volume) in 20 patients treated for 1 year.20,21
These positive results have led to the planning of two new US studies that are currently underway: the TSC Multicenter Clinical Trial, designed to evaluate the efficacy of rapamycin in the treatment of AML through the control of the evolution of other manifestations, and the MILES study, which will evaluate the effects of the drug on sporadic lymphangioleiomyomatosis and those cases associated with TS in 240 patients. In Europe, the TESTAL study will measure the effects of rapamycin on the size of AML in TS patients. So far, this study, which is being performed in Great Britain, has included 12 patients.
In this study, we have shown that the treatment of patients with TS using sirolimus produces a >50% decrease in AML volume after 12 months of treatment.
We believe that this decrease in volume is due to a double effect of rapamycin: growth inhibition due to the direct antiproliferative effect of the drug, and indirectly through the inhibition of angiogenesis.
We have observed that the greatest decrease in AML volume is produced during the first 6 months of treatment, and is probably due to the initial inhibition of mTOR and the greater initial volume of the AML at the start of treatment. Later, with the maintained inhibition of mTOR, we observed a lower rate of decrease in volume and a stabilisation in AML size, which leads to the inference that, on a long-term basis, perhaps it is not necessary to maintain high doses of the drug in order to sustain the inhibition of mTOR.
The most frequent adverse reactions that we observed were the appearance of stomatitis (5/17), observed at the start of treatment, which was dosage-dependent and easily controlled using topical corticosteroids and adjusted treatment doses, followed by hypertriglyceridemia (3/17), observed in patients that already had levels in the upper limit before inclusion in the study, which required medical treatment. We also observed microcytosis and hypochromia (3/17) due to the antiproliferative effect of rapamycin, although results from the iron metabolism analysis were within normal values and haemoglobin levels were stable. Lastly, we observed one case of diarrhoea (1/17) and one of acne (1/17), which were caused by the rapamycin.
No patients developed deteriorated kidney function.
Two patients were removed from the study, one due to reactivation of an erythema nodosum (1/17) that was already present before treatment, and another due to the appearance of nephrotic proteinuria (1/17), which was resolved after treatment suspension.
The results from our study show that mTOR inhibitors are a safe and effective alternative for the treatment of AML in patients with TS. This is a less aggressive medical treatment than the currently available options, and reduces the size of AML and thus the risk of haemorrhage.20
Given the efficacy and acceptable safety profile of mTOR inhibition in patients with TS, we expect the studies currently underway to confirm these findings and support the use of these drugs as a useful therapeutic option in the treatment of TS.
CONCLUSIONS
1. The mTOR hamartin-tuberin signalling pathway is a valid and effective treatment target in patients with TS.
2. Treatment with sirolimus during one year reduces AML size by over 50% in patients with TS.
3. Given the presence of adverse effects and the methodological limitations presented by our study, since this is a study with no controls and a limited number of patients due to the low prevalence of the disease, we believe that multicentre studies are needed that evaluate the long-term efficacy and safety of using rapamycin to treat patients with TS.
4. However, since this is a rare disease with no other pharmacological alternative currently available, our promising short-term results appear to offer an effective and less aggressive therapeutic alternative in the treatment of AML in patients with TS.
Table 1. Evolution in size of angiomyolipomas
Table 2. Adverse effects
Figure 1. Reduced volume of angiomyolipomas
Figure 2. Percent reduction of angiomyolipoma volume
Figure 3. Mammalian target of rapamycin cascade (mTOR)