El fracaso renal agudo en el mieloma múltiple (MM) ocurre en un 12-20 % y es un factor de mal pronóstico para la supervivencia del paciente. Estudios recientes muestran que la diálisis con membrana «High-Cut-Off» (HCO) depura eficazmente las cadenas ligeras libres (CLL), aunque con gran pérdida de albúmina. Otras técnicas basadas en la adsorción, como la hemodiafiltración con regeneración del ultrafiltrado mediante adsorción en resina (HFR SUPRA), no han sido estudiadas. Se presentan tres casos de MM, dependientes de hemodiálisis desde el diagnóstico: dos son IgG kappa y uno IgA lambda. Los tres recibieron quimioterapia y HFR SUPRA. El objetivo del estudio fue evaluar la eficacia de la HFR SUPRA en la reducción de CLL, así como su efecto sobre la albúmina. Se obtuvieron muestras sanguíneas pre y posdiálisis y muestras de ultrafiltrado (UF) pre y posresina a los 5 minutos de empezar la sesión y 5 minutos antes de finalizar. La tasa de reducción media por sesión de CLL en sangre en los tres pacientes fue del 53 % y del 63 % (kappa) y del 38 % (lambda). En el UF la tasa de reducción media de CLL fue cercana al 99 %, tanto al inicio como al final de la diálisis, sin eliminación de albúmina. Con los resultados obtenidos podemos concluir que con esta técnica se consigue una reducción eficaz de las CLL, que se mantiene durante toda la sesión, sin que se produzca saturación de la resina y sin pérdida de albúmina. Por tanto, la HFR SUPRA es eficaz como tratamiento coadyuvante del MM.
Acute kidney failure in multiple myeloma (MM) occurs in 12%-20% of patients and is a poor prognostic factor for patient survival. Recent studies have shown that dialysis with a High-Cut-Off membrane (HCO) removes free light chains (FLC) effectively although with significant albumin loss. Other adsorption-based techniques, such as haemodiafiltration with ultrafiltrate regeneration by adsorption in resin (SUPRA-HFR), have not been studied. We present three cases of MM, all haemodialysis-dependent since diagnosis. Two cases were IgG kappa and one was IgA lambda. All patients were treated with chemotherapy and SUPRA-HFR. The aim of this study was to evaluate the effectiveness of SUPRA-HFR in the reduction of FLC and its effect on albumin. We collected blood samples pre- and post-dialysis, and ultrafiltrate (UF) samples pre- and post-resin 5 minutes into the session and 5 minutes from the end. The mean reduction rate of FLC in blood per session in the three patients was 53% and 63% (kappa) and 38% (lambda). In the UF, the mean FLC reduction rate was close to 99%, both at the start and at the end of dialysis, without the removal of albumin. With the results obtained we can conclude that this technique achieves an effective reduction of FLC, which is maintained throughout the session, without resin saturation and without albumin loss. Therefore, SUPRA-HFR is effective as an adjunctive therapy for MM.
INTRODUCTION
Multiple myeloma (MM) is a clonal proliferation of plasma cells that is characterised by excessive production of a certain type of immunoglobulin or fraction thereof. Acute renal failure (ARF) occurs in 12-20% of cases and is a poor prognostic factor for patient survival,1,2 probably because it indicates that the disease is more aggressive and has more complications.3-5 Approximately 10% of patients with ARF due to MM require haemodialysis (HD)3,6 and the mean one-year survival rate in this case varies between 30% and 84%.7 Furthermore, mean survival in chronic HD patients with myeloma is 11 months.8
The most common cause of ARF is the excessive production of free light chains (FLC), which cause a cast nephropathy, known as "myeloma kidney". These casts are composed of cell fragments, FLC and Tamm-Horsfall proteins. Most of the casts cause, at a microscopic level, obstruction of the distal tubule lumen and frequently induce a local inflammatory reaction with the appearance of multinucleated giant cells typical of myeloma kidney.9
The objective of treating MM is to reduce or eliminate FLC production with chemotherapy (dexamethasone, bortezomib, melphalan, cyclophosphamide, thalidomide, lenalidomide) and/or autotransplantation of haematopoietic cells.9,10 As adjunctive treatment for reducing renal damage associated with FLC, in recent years, extracorporeal clearance techniques have been employed that reduce their concentration in plasma. The effectiveness of plasmapheresis in this type of patient is uncertain.11 In 2005, Clark published a randomised controlled trial on 104 patients with MM and ARF, in which no substantial clinical benefits after treatment with plasmapheresis were found.12 In recent years, studies have been published on the effectiveness of very high permeability "High-Cut-Off" (HCO) membranes in the removal of FLC. HCO membranes have a large pore with a cut-off of 45-60kD, thus allowing filtration of kappa and lambda FLC, which have a molecular weight of 22.5kD and 45kD, respectively.3,13,14 A high removal of FLC is achieved with this membrane, which is more effective the earlier the diagnosis and treatment of MM.3,15 The first studies published obtained good results using long daily HD sessions (8 hours) with HCO. With this regimen, a sustained reduction of light chains is obtained, with a renal function recovery rate of up to 60%.10,16 Furthermore, there is a linear relationship between early treatment and the rate of renal function recovery, which is associated with survival.17 The main drawback is that HCO membranes cause a substantial loss of albumin, and therefore subsequent albumin replacement is required.10,16 Another issue to consider is its high price.
Recently, SUPRA-HFR (haemodiafiltration with regeneration of the ultrafiltrate by adsorption in resin) has been introduced as an extrarrenal clearance technique that combines convection, adsorption and diffusion. It uses a dual chamber dialyser: the first with a “Super High Flux” polyphenylene membrane that has a cut-off of 42kD through which ultrafiltration is performed, and the second with the same membrane, but of low permeability, where diffusion is carried out. The ultrafiltrate (UF) obtained in the first chamber passes through a resin cartridge where adsorption occurs and it is reinfused before the second chamber. This technique is used in HD patients due to its high protein-bound toxin adsorption capacity, with the great advantage that albumin is not removed.18,19
Since the cut-off of 42kD theoretically allows the passage of FLC, especially kappa FLC, HFR might be useful for removing FLC, as a chemotherapy aid in MM with associated ARF. A recent study has shown that HFR removes FLC effectively, particularly kappa FLC, in dialysis patients with both monoclonal and polyclonal gammopathies20 and our group has obtained very promising preliminary results in a patient with ARF due to MM requiring HD.21
The objective of this study was to evaluate the effectiveness of SUPRA-HFR in reducing FLC and albumin in three patients with MM and ARF due to myeloma.
METHODS
We present three patients with renal failure secondary to MM diagnosed in our centre between July 2012 and January 2013. The first two had ARF due to IgG kappa MM and the third had chronic kidney disease (CKD) that was exacerbated due to IgA lambda MM.
Patients
Case 1
A 63-year-old female, without relevant medical history, was admitted with ARF secondary to IgG kappa MM dependent on renal replacement therapy (RRT) from diagnosis. Diagnosis was performed by bone marrow aspiration, in which we observed 90% of plasma cells. Although we took blood and urine samples to measure FLC, these could not be tested until two months after starting treatment. We were we unable to perform a renal biopsy. She initially received bortezomib and dexamethasone, and since FLC levels were not available, we dismissed special clearance techniques, starting online haemodiafiltration four times a week with 240 minute sessions. The patient maintained residual diuresis of 1000cc/24h and plasma creatinine values of around 5mg/dl. When we were able to measure FLC, we observed that the figure was 18,806mg/l (FLC in urine: 77mg/l, kappa/lambda: 8593.9) and that she had not responded to chemotherapy, remaining at around 17,422mg/l two months after treatment. As such, we treated her with cyclophosphamide. At that time, we decided to start the patient on 240 minute SUPRA-HFR sessions three days a week, but after the first dose of cyclophosphamide, the FLC had declined notably (from 17,422mg/l to 761mg/l after 14 days). The preliminary results of this case have previously been published separately.21
Case 2
A 52-year-old female with a history of high blood pressure and vertebral compression fractures, on treatment with non-steroidal anti-inflammatory drugs and with previous normal renal function. She was admitted to the Haematology department due to anaemia within transfusion range and ARF (urea 192 and creatinine 12.5mg/dl) and was diagnosed with IgG kappa MM. Bone marrow aspiration had 33% of plasma cells, plasma FLC was 6178mg/l and urine FLC was 135mg/l (kappa/lambda: 440.9). We were not able to perform a renal biopsy because of poor patient cooperation due to her vertebral compression fractures. Following diagnosis, chemotherapy was started with thalidomide, bortezomib and dexamethasone, and RRT with SUPRA-HFR, initially with 180 minute daily sessions, and a progressive reduction in the number and duration of sessions, depending on FLC plasma levels and renal function progression. She maintained residual diuresis of around 800cc, which subsequently increased.
Case 3
A 79-year-old male with a history of high blood pressure, bilateral hearing loss, benign prostatic hyperplasia, chronic obstructive pulmonary disease and CKD of unknown aetiology for 1 year with baseline creatinine of 1.7mg/dl. He was admitted due to exacerbation of CKD with creatinine of 4.3mg/dl and was diagnosed with IgA lambda MM. Bone marrow aspiration had 14% of plasma cells and plasma FLC was 1325mg/l (kappa/lambda: 0.01). We were unable to perform renal biopsy due to a small left kidney (8.5cm), with cortical thinning, which was very echogenic and very suggestive of chronic nephropathy. At this time we began chemotherapy with bortezomib and dexamethasone and given the deterioration in renal function, we started RRT with SUPRA-HFR 13 days after admission, with a regimen of 240 minute sessions, three days a week. The patient was oliguric.
Clearance technique
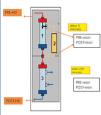
SUPRA-HFR (Bellco©) is an extrarrenal clearance technique that combines convection, adsorption and diffusion. It uses two filters, the first of which has a “Super High Flux” (SHF) Purema® polyphenylene membrane, with a larger pore than other SHF used in haemodiafiltration (cut-off of 42kD) and a surface of 0.7m2. The second filter is a low permeability polyphenylene filter with a 1.7m2 area. Firstly, a convective process is performed, with generation of a UF that passes to the adsorbent resin cartridge (Suprasorb, 80ml) at a flow rate of up to 70ml/min, where toxins are released. The UF is subsequently reinfused before the second filter, which is where the diffusion process takes place (Figure 1).
Blood flow was between 300 and 350ml/min, dialysate flow was 500ml/min and the ultrafiltration rate changed depending on the patients’ interdialysis weight gain. We used a calcium concentration of 3mEq/l and a potassium concentration of 2mEq/l in the dialysate. The number of sessions and their duration was adjusted individually in each case. The extracorporeal circuit was anticoagulated with an initial bolus of heparin sodium and an hourly dose. Initial vascular access was performed with a temporary catheter that, after two weeks of treatment, was replaced with a permanent catheter. Given that HFR is only moderately more expensive than other haemodiafiltration techniques, treatment was extended while the patient required dialysis, even if the light chains were less than 500mg/l.
Laboratory tests
We obtained blood samples from the arterial line at the beginning and end of dialysis and UF samples before and after the resin cartridge, 5 minutes into the session and 5 minutes before the end (Figure 1). As such, we removed 6 aliquots per session (2 from blood and 4 from UF) and we measured FLC and albumin in each. The extractions were performed weekly, on the first day of dialysis.
Albumin in blood and UF was measured by the bromocresol purple colorimetric enzymatic method. For independent quantification of kappa and lambda FLC, both in blood and UF, we used a Freelite kit (The Binding Site) for use in the Siemens BN® II nephelometer, a test validated for renal failure patients.22 The normal range with this technique is 3.3-19.4mg/l for kappa and 5.71-26.3mg/l for lambda.
RESULTS
All patients received chemotherapy, in accordance with Haematology protocol and RRT with SUPRA-HFR, with indication of the number of sessions on an individual basis. Cases 1 and 3 underwent HFR sessions 3 days a week (with a total of 59 and 30 sessions, respectively), while case 2 initially underwent daily sessions with a progressive decrease in time and the number of sessions in accordance with renal function and plasma FLC levels (66 sessions in total).
Removal of free light chains and albumin in blood
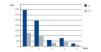
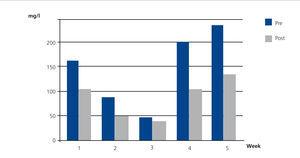
Case 1 was ARF due to IgG kappa MM with poor initial response to haematological treatment. When we decided to start SUPRA-HFR, kappa FLC levels were 105.19mg/l, although from week 16 we observed an increase in the latter to 700mg/l. The mean reduction rate throughout treatment was 63.4%. Blood albumin concentration did not change (Figure 2).
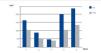
Case 2 was ARF due to IgG kappa MM with FLC levels prior to the first HFR session of 3642mg/l. From the third week of treatment we observed plasma FLC figures that fluctuated around 500mg/l, and lower figures were maintained from the fifth week. The mean rate of reduction per session in this patient was 53.8%. Blood albumin slightly increased after dialysis, probably due to haemoconcentration (Figure 3).
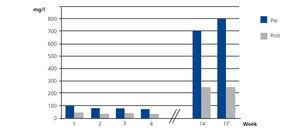
Case 3 was CKD exacerbated by IgA lambda MM with FLC levels of 160.4mg/l before beginning treatment with HFR. In this case, the mean reduction per session of lambda FLC was 38% and albumin also increased slightly (Figure 4).
We observed that the rate of FLC reduction was related to their plasma level and as such, as their concentration decreased, we obtained a lower reduction percentage.
Removal of free light chains and albumin in ultrafiltrate
In UF, the mean rate of FLC reduction was much higher than in blood. In case 1, the mean rate of kappa FLC reduction in UF at the start of the session was 98% and was 79% 5 minutes before the end. With regard to albumin, its UF concentration was 0.4g/dl before resin and 0.4g/dl after it, both 5 minutes after starting the session and 5 minutes before the end.
In case 2, the mean rate of kappa FLC reduction was 99% at the start and 58% at the end. Albumin UF concentration was 0.5g/dl before resin and 0.5g/dl after resin at the start and end of the session.
In case 3, the mean rate of lambda FLC reduction was 99% 5 minutes into the technique and 72% 5 minutes before the end. In this case, we also detected very little albumin in the UF both at the start and the end of the session.
Patient’s progression
In case 1, when we started treatment with SUPRA-HFR, kappa chain levels were already 105mg/l. In spite of treatment, the patient’s renal function did not recover. An autotransplant of hematopoietic cells from peripheral blood was performed and she died ten days later due to septic shock.
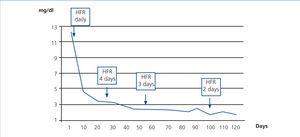
In case 2, treatment with SUPRA-HFR was started early. Three weeks after starting treatment, FLC figures were less than 500mg/dl and creatinine was around 3mg/dl, and as such, we reduced the number of FLC HFR sessions to three days a week, with 240 minute sessions. After three months, FLC had normalised and the patient discontinued dialysis, remaining stable with creatinine of 1.9mg/dl eight months later (Figure 5).
Case 3 was not ARF secondary to MM, but rather was exacerbated CKD due to MM and, furthermore, it was IgA lambda. We started RRT with SUPRA-HFR three days after receiving the first dose of bortezomib, but there was no improvement in renal function and the patient remained dependent on HD. He remained alive 6 months after diagnosis.
There were no complications associated with the technique in any case. Neither were there changes in levels of phosphorus, potassium or magnesium.
DISCUSSION
Our study demonstrated that with the SUPRA-HFR dialysis technique, an effective removal of FLC is achieved and is maintained throughout the session, without resin saturation or loss of albumin. When combined with chemotherapy, SUPRA-HFR is effective as an adjunctive treatment for MM and allows the recovery of renal function, as occurred in the second case in our small series. In our patients, we obtained a mean plasma FLC reduction rate of 53% and 63% respectively in all kappa MM cases and 38% in lambda MM. This reduction percentage was the mean of all sessions carried out on each patient. We observed that in the first sessions, FLC reduction was much higher and as their plasma levels decreased, the reduction percentage decreased. We must highlight that in case 3 (lambda FLC), we started with very low baseline levels, and as such, we did not achieve a high reduction percentage. Furthermore, lambda FLC have a molecular weight that is somewhat higher than the SHF polyphenylene membrane cut-off (42Kd), in spite of which, we achieved a mean reduction of 38%.
In recent years, we have been testing different extracorporeal clearance techniques to reduce plasma FLC concentration and, therefore, decrease renal damage. The first attempts to remove FLC were carried out with plasmapheresis. In 2005, Clark published a randomised controlled study on 104 patients with MM and ARF. This study did not show clinical benefits (substantial reduction in mortality, dialysis dependence or improvement in the glomerular filtration rate) after treatment with plasmapheresis combined with chemotherapy.12 By contrast, in 2008, Leung observed that, out of 40 patients with cast nephropathy, diagnosed by biopsy and treated with plasmapheresis, in 75%, nephropathy was resolved when plasma FLC reduced by more than 50%. When FLC reduction was less, there was no clinical response.11 In our cases, SUPRA-HFR achieved a reduction in the plasma kappa FLC figure of more than 50% in the two patients with IgG kappa myeloma and this without a doubt contributed to the recovery of renal function in patient 2. The main disadvantage of plasmapheresis is the very high cut-off of the plasma filters, and as such, high rates of plasma exchange, such as those that are required to remove FLC, which are mainly extravascular, are associated with the loss of albumin and other proteins with a high molecular weight that are essential for the body. This means that it is practically impossible to use the treatment for an extended period of time. Recently, several studies have been published on the effectiveness on FLC removal in very high permeability HCO dialysis membranes designed for this purpose. For the first time in 2007 Hutchison et al. published that the HCO membrane removes a significantly higher amount of both types of FLC than six other dialysers studied, including polymethylmethacrylate (PMMA).10 These same authors treated 13 patients with extended HCO dialysis for eight hours, in combination with a bortezomib-based chemotherapy regimen and they achieved a mean plasma FLC reduction rate per session that fluctuated between 69% for kappa and 71% for lambda.16 When HCO dialysis was used in patients with dialysis-dependent ARF due to MM in combination with bortezomib-based chemotherapy regimens, renal function recovery rates of 60%-74% were achieved.16,23-25 In our study, patient 1 achieved a reduction of 63.4% and patient 2 of 53.8%, figures that were only slightly lower than those reported with HCO for kappa FLC. Patient 3 had an IgA lambda MM and in this case, the reduction percentage was 38%, clearly lower than the kappa reduction percentage. This was due, on one hand, to the molecular weight of lambda FLC being somewhat higher than the cut-off of the polyphenylene dialyser used in SUPRA-HFR, and on the other hand, this patient began with a figure of 160.4mg/l, which was not excessive. Of the three patients, patient 1 had a poor response to bortezomib, she only responded partially when cyclophosphamide was added and treatment with SUPRA-HFR was introduced two months after the start of ARF. The third patient had a CKD that was exacerbated by myeloma and, furthermore, the FLC figure was low when RRT was required. Only patient 2 discontinued dialysis and their renal function recovered practically ad integrum. Many favourable circumstances combined in this patient: HFR treatment was started quickly after diagnosis, the response to chemotherapy was good and the kappa FLC figure was less than 500mg/l from the third week of treatment. Recently published studies show that dialysis with HCO membranes is more effective the earlier the diagnosis and treatment of MM,3 which may be extended to other membranes or dialysis techniques. Furthermore, there is a linear relationship between the FLC reduction rate and renal function recovery, such that 60% after 21 days of treatment is associated with renal function recovery in 80% of patients.17 In later studies, on a smaller number of patients, similar results were obtained, although a higher rate of FLC reduction was not directly associated with renal function recovery.25
Some adsorption-based extrarrenal clearance techniques may eliminate a large amount of FLC without the disadvantage of albumin loss and at a lower cost than HCO membranes. Testa et al. demonstrated in 2010 in a group of CKD patients on dialysis and monoclonal gammopathy that HFR is capable of reducing the kappa FLC figure by 30-35%. In this study, they used a superflux polyphenylene membrane with a smaller surface and a cartridge with a smaller amount of resin than SUPRA, which explained the lower FLC reduction percentage. With SUPRA-HFR, the FLC that were obtained in the UF were almost completely absorbed in resin with a 99% reduction being obtained in the UF at the start of the session and 58%-79% at the end, which indicated that the resin was hardly saturated.
The adsorption properties of the PMMA membrane have recently been studied and it has been proven that it produces a 31% reduction in kappa FLC and a 53% reduction in lambda FLC, using dialysers in series, which were changed every 2 hours26 or by one circuit with two filters in series.27 The reduction of FLC with PMMA dialysers occurs mainly due to their adsorption into the membrane.10 Although it is a cheap technique, in which albumin is not lost, it seems that FLC removal is lower than that obtained with SUPRA-HFR and HCO.
An advantage of SUPRA-HFR compared with HCO extended dialysis is that with the latter, a considerable amount of albumin is lost that it is necessary to replace at the end of the session. Depending on the number of filters used there is an average loss of serum albumin of 3.9g/l.10 The albumin replacement that is required for 8 hours of HD using a dialyser is 12g and with two dialysers it is 45g,14 which involves an additional cost for this technique. With SUPRA-HFR we found a minimum quantity of albumin in the UF, which is returned to the patient after passing through the resin, and as such, the figure in blood was not modified.
Another major advantage of SUPRA-HFR is its low cost compared to dialysis with HCO. A study recently compared the cost-effectiveness of myeloma kidney treatment with that of conventional HD and HCO, taking renal recovery into account, and the use of HCO involved annual savings of 6000 pounds sterling per patient (more than 7000 euros).28 In this study, it was considered that an HCO filter has a cost of approximately 600 euros (1200 per session if two filters are used) and replaced albumin around 75, and as such, each session costs around 1300 euros, without taking into account healthcare staff expenses. The SUPRA-HFR session costs around eight or nine times less, including the dual filter, the resin cartridge and the lines, since with this technique it is not necessary to replace albumin. For this reason, HFR treatment was extended in the three cases while the patient required dialysis, even if the light chains were less than 500mg/l, since this technique is only moderately more expensive than other haemodiafiltration techniques. New studies that compare the effectiveness of both techniques with regard to renal function recovery and their costs will be necessary.
It is necessary to add to all of the above the effect of HFR on inflammation, which could be beneficial for MM progression. Recent studies demonstrate that HFR reduces inflammation markers, such as tumour necrosis factor alpha, interleukin 6 and C-reactive protein, as well as oxidative stress.29,30
The main disadvantage of SUPRA-HFR is that its effectiveness in the removal of lambda light chains is lower than that of HCO, and as such, in our opinion, it should be used mainly in patients with MM with kappa FLC.
The main limitation of our study is the small number of patients treated. To compensate for this deficiency, at least in part, we measured FLC before and after dialysis and before and after resin at the beginning and at the end of the session at least once a week for at least 16 weeks. This minimised potential anomalies in the values measured and allowed us to obtain a good FLC reduction profile over successive sessions. Another limitation was the impossibility of performing a renal biopsy in the three patients. Although the extrarrenal clearance techniques are only effective in light chain nephropathy and the latter can only be diagnosed by biopsy, it is not always possible to carry it out, as was the case for our patients. Although in the first studies published a biopsy was an essential requirement to include for patients, in a recent multicentre study led by Hutchison, only 56.7% of patients had a renal biopsy and cast nephropathy was the predominant diagnosis (86.7%).24 We believe that renal biopsy should always be performed whenever possible, not only in order to establish the diagnosis, but also the prognosis, but, if it is not possible, patients with MM and renal failure must be treated with techniques that reduce plasma FLC concentration.
To conclude, SUPRA-HFR is a dialysis technique that provides a significant and sustained reduction in plasma FLC in patients with MM and ARF, without the loss of albumin and at a low economic cost. When combined with effective chemotherapy and when used early, it is possible for these patients to recover from renal failure.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Figure 1. Haemodiafiltration diagram with ultrafiltrate regeneration through adsorption in resin.
Figure 2. Free kappa light chains in serum before and after haemodiafiltration with ultrafiltrate regeneration (HFR) (case 1).
Figure 3. Free kappa light chains in serum before and after haemodiafiltration with ultrafiltrate regeneration (HFR) (case 2).
Figure 4. Free lambda light chains in serum before and after haemodiafiltration with ultrafiltrate regeneration (HFR) (case 3).
Figure 5. Progression of serum creatinine in relation to haemodiafiltration sessions with ultrafiltrate regeneration (HFR) (case 2).