Introducción y objetivos: El tratamiento de la anemia en hemodiálisis (HD) con hierro y agentes estimulantes de la eritropoyesis (AEE) no siempre consigue un control adecuado de esta, habiéndose relacionado con la inflamación. El efecto antiinflamatorio de la pentoxifilina (PTX) podría resultar beneficioso en estos casos. El objetivo fue estudiar el posible efecto de la PTX en la anemia de pacientes en hemodiálisis. Pacientes y métodos: Estudio observacional retrospectivo de 18 casos (reciben PTX) y 18 controles (sin PTX, emparejados por edad y sexo) en HD (Clínica Universidad de Navarra). Cuatro pacientes tomaban PTX por vasculopatía y 14 por anemia resistente (definición: hemoglobina [Hb] < 11 g/dl en los últimos 3 meses a pesar de dosis altas de AEE y saturación de transferrina > 20 %). Hb, dosis de MIRCERA® y proteína C reactiva fueron recogidas antes del tratamiento con PTX (basal), a los 3 meses y al final del seguimiento. Resultados: En los pacientes con PTX hubo aumento de la Hb (p < 0,001) en 3 meses y descenso de las dosis de AEE al final del estudio (p = 0,002). Las diferencias basales en Hb (menor en casos) (p < 0,001) y en dosis de AEE (mayor en casos) (p = 0,006) entre grupos desaparecieron a los 3 meses. Al final del estudio 11/18 (61,1 %) de los tratados con PTX tenían Hb en rango deseado y recibían dosis de AEE equiparables al control. Conclusiones: PTX en dosis de 800 mg/día mejora significativamente y a corto plazo (3 meses) la Hb de pacientes en HD (respuesta en torno al 61 %) y permite la reducción de dosis de AEE a medio-largo plazo. El efecto se mantiene en el tiempo y el tratamiento es bien tolerado.
Introduction and objectives: Treatment of anaemia in haemodialysis (HD) with iron and erythropoiesis-stimulating agent (ESA) does not lead to adequate anaemia control and has been associated with inflammation. The anti-inflammatory effect of pentoxifylline (PTX) may be beneficial in these cases. Our aim was to study the potential effect of PTX on anaemia in haemodialysis patients. Patients and method: Retrospective observational case-control study on 18 patients (treated with PTX) and 18 controls (without PTX matched by age and sex) on HD (Clínica Universidad de Navarra). Four patients received PTX due to vascular disease and 14 due to refractory anaemia (which was defined as haemoglobin [Hb] <11g/dl in the last three months despite high doses of ESA and transferrin saturation of >20%). Hb, MIRCERA® dose and C-reactive protein were recorded before starting PTX treatment (baseline), at 3 months and at the end of the study. Results: In patients who received PTX, there was an increase in Hb (P<.001) over three months and a decrease in the ESA dose at the end of the study (P=.002). The baseline differences in Hb between groups (lowest of all cases) (P<.001) and ESA dose (highest of all cases) (P=.006), disappeared at 3 months. At the end of the study, 11/18 (61.1%) of patients treated with PTX had adequate Hb levels and received doses of ESA comparable with those of the controls. Conclusions: In HD patients, PTX in doses of 800mg/day improves Hb significantly and in the short term (3 months) in HD patients (around 61% response) and allows the required ESA dose to be reduced in the medium-long term. This effect is sustained over time and treatment is tolerated well.
INTRODUCTION AND OBJECTIVES
Anaemia, a complication of chronic kidney disease (CKD) attributed to erythropoietin (EPO) deficiency, has an incidence of over 70% in advanced stages of the disease.1 Medical treatment includes administration of erythropoiesis-stimulating agents (ESA) and periodic iron supplements. The high rate of response to this treatment has meant that it has been possible not only to avoid red blood cell transfusion in patients on haemodialysis (HD), but also improve their quality of life substantially. However, since EPO started to be used, it has been observed that there is a group of patients with resistance to ESA. In some cases it is attributable to easily treatable factors, such as iron deficiency, severe hyperparathyroidism, ineffective dialysis, blood loss, vitamin deficiencies, etc.; and in others, inflammation has been suggested as a mechanism involved.2,3 In fact, all dialysis patients have a certain degree of inflammation that has been related to a greater or lesser extent, to anaemia, according to the cases.2,4 Inflammation may result in, not only a lower production of EPO, but also a lower response of the erythropoiesis progenitor cell to the aforementioned treatment.5
Pentoxifylline (PTX), derived from methylxanthine, is a non-specific inhibitor of phosphodiesterases that, besides having rheological properties6 and being used as a treatment in peripheral vascular disease, has anti-inflammatory activity. In fact, it has been described that it reduces levels of interleukin-6 (IL-6) and other inflammatory parameters in patients on HD.7 As for the possible benefit of its anti-inflammatory action on anaemia in renal patients, some studies show that it increases haemoglobin (Hb) in patients with CKD,8-10 but there are no clear supporting data in patients on a regular HD programme.11
The main aim of this study was to evaluate the change in Hb levels and the dose of ESA in HD patients treated with PTX, compared to a control group without the aforementioned treatment.
The secondary aims were: 1) to study levels of C-reactive protein (CRP) as a marker of inflammation in these patients; and 2) to analyse the tolerance to and safety of PTX in the treated group.
MATERIAL AND METHOD
Patients
We studied 36 patients on a regular HD programme of the Clínica Universidad de Navarra, Pamplona, of which 18, considered cases, received treatment with PTX, and 18 did not (controls). The inclusion of patients was carried out in March 2011, after obtaining informed consent and the approval of the study by the hospital’s ethics committee.
There were 2 inclusion criteria for all (cases and controls): age over 17 years and being on a regular HD programme for at least three months before the study. Cases also received oral PTX. We recorded whether the reason for taking PTX was peripheral vascular disease or resistance to standard anaemia treatment. The ESA used to treat anaemia, both in cases and controls was MIRCERA® (methoxy polyethylene glycol-epoetin beta). The lack of response to treatment of anaemia in our unit was defined as Hb levels below 11g/dl in the last three months despite our patients having received ESA doses greater than 1.5 times the standard dose (standard dose: 100μg/month) and having a transferrin saturation index (TSI) above 20%. Exclusion criteria were: diagnosis of neoplasia or haematological disease, steroid treatment, major surgery, haemorrhage, prolonged hospital stay (exceeding 15 days), blood product transfusion in the last three months, changes in the frequency or type of dialysis during the observation period and frequent symptomatic hypotension in HD (more than 1 session/week).
All patients were dialysed with an average permeability helixone membrane (1.4 or 1.8m2, depending on the cases), dialysis flow of 500ml/min, and bicarbonate. Furthermore, all were treated with a low dose angiotensin II converting enzyme inhibitor (ACEI) and a HMG-CoA reductase inhibitor (statin), as part of the standard protocol of our HD unit.
Method
This is a retrospective observational case-control study of cases and controls in which data was collected and analysed in March-April 2011. Patients included were studied between August 2008 and March 2011, which was a long period of time due to different treatment start dates in each case. Controls were selected from the same HD unit as those treated, and were matched by sex and age. From the medical history of all patients (cases and controls), the following data were collected (on the same dates for cases and controls): Hb level, MIRCERA® dose, CRP and intact parathyroid hormone (PTH) levels. These data were collected at three different times: before starting treatment with PTX, at three months and at the end of the follow-up period (when patients were included). At the beginning and the end of the observation period, ferritin levels were also recorded. The mean baseline TSI for patients taking PTX due to refractory anaemia was 35.5% (standard deviation [SD]: 2.9). No TSI progression data were available (at three months and at the end of the study) for any patient.
The following variables were also collected for each patient: dry weight, body mass index (BMI), volume of distribution calculated by the Watson formula,12 Charlson’s comorbidity index, diabetes, cause of CKD, type of vascular access, HD modality, the frequency and duration of sessions, time on HD and, as a dialysis efficacy parameter, the Kt value.
Statistical analysis was carried out using the SPSS v17 software (SPSS Inc, Chicago), and we considered P<.05 to be significant. The quantitative variables were expressed as mean and SD, and the qualitative variables as a number and percentage. The comparison between groups was carried out by the Student’s t-test or the Χ2 test, according to the type of variable. To assess the progression of variables studied over time (baseline, at three months and end) a repeated measures linear model was used.
RESULTS
The inclusion of patients was conducted in March 2011. The time range in which the 18 patients treated to that date received PTX was between 6 and 32 months, with a mean total follow-up time of 16.6 months. Of the 18 patients, 4 received PTX due to vascular disease and 14 due to anaemia that did not respond adequately to normal treatment. The most frequent PTX dose regimen both at the start and the end was 400mg/12 hours. At these doses, there were no adverse effects or intolerances that may have resulted in a dose reduction or discontinuation of the drug.
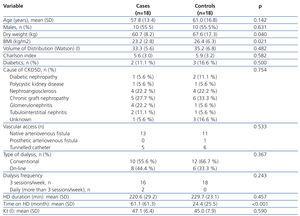
The demographic and clinical characteristics of cases and controls, as well as dialysis-related variables are described comparatively in Table 1. There were no differences in terms of age, sex, diabetes frequency, cause of CKD, vascular access type, dialysis modality, frequency and duration of the HD session or in the Kt value. Mean Kt values were observed in both groups as being at the lower limit of normal. This fact can be explained if we consider that the mean duration of the HD session was below 240 minutes because some patients (2 cases and 4 controls) over 75 years of age, and more associated comorbidities did not accept a longer period of dialysis. Values for dry weight (P=.04) and BMI (P=.021) were significantly lower in the treated group. However, since the mean BMI (23.5 kg/m2) did not correspond to a malnutrition value, it does not appear that this could have had an influence on anaemia control in these patients. The time on dialysis was significantly higher in the treated group (P<.001), with this being a purely observational finding (cases and controls were only matched for age and sex), but one which is of some interest in the discussion of the results.
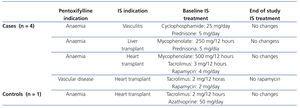
Of the 36 patients included, although in 11 (5 cases and 6 controls) the cause of CKD was renal graft loss, the results are not influenced by the taking of immunosuppressants or graft-associated inflammation for the following data: of the 5 controls, 3 no longer had a graft (transplantectomy) and of the other 2, only one was taking steroids and the other did not take immunosuppressants, due to their dialysis treatment for 10 months. As for the 6 controls, 3 no longer had a graft (transplantectomy) and the other 3, who had been on dialysis for over 6 months, were without immunosuppression. On reviewing the treatments received by the remaining patients, we found that during the study period, 4 cases and 1 control were taking immunosuppressants for various reasons. Table 2 includes for those patients the reason for taking PTX, the immunosuppressive treatment indication, drugs with their doses, as well as the changes recorded in treatment during the study. Of these, only one case (heart transplant) had a change in the immunosuppressive regimen, noting that in the three month control, he no longer took rapamycin. However, this patient had been started on PTX due to peripheral vascular disease, and not to optimise the control of refractory anaemia.
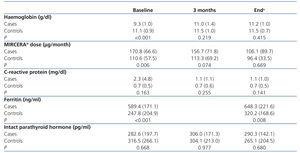
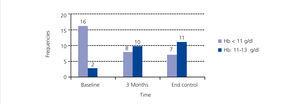
The change in Hb levels, CRP, MIRCERA® dose, ferritin and intact PTH by groups is included in Table 3, with differences between groups (case-control). With regard to the intragroup differences for the variables analysed, it was observed that in the treated group, there was a significant increase in Hb at three months (P<.001) and at the end of the study (P<.001) compared to baseline. In the same group, there was a non-significant decrease in the ESA dose at three months (P=.226), but this reached statistical significance at the end of the study (P=.002). However, when a numerical decrease was observed in the ESA dose in the control group throughout the study, this decrease was not significant (p>.05). Neither were there any significant differences in time for the rest of the variables in both cases and controls. We studied the number of patients treated with PTX who reached the recommended Hb level (11 to 13g/dl) at the different times of study: baseline (before starting treatment), at three months and at the end of follow-up. Figure 1 shows the results, and it can be observed that, while at the start of treatment with PTX, only 2 of 18 (11.1%) were in the target Hb range (11-13g/dl), at three months, the figure was 10 out of 18 (55.5%) and at the end of follow-up it was 11 out of 18 (61.1%). In no case was the recommended maximum value for Hb exceeded. Of the 7 who did not reach the recommended level of Hb with PTX with respect to the 11 that did, there was no difference in frequency of diabetics and peripheral vascular disease (none of the 7 who did not reach the recommended level of Hb were diabetic or had peripheral vascular disease), tunnelled central venous catheter (28.6% in those who did not respond compared to 27.3% in those who did, P=.676) and on-line HD (57.1% in those who did not respond compared to 36.4% in those who did, P=.352). However, in the group that did not achieve the recommended Hb range, 49.2% were taking immunosuppressants, compared to 9.1% in the group that did achieve it (P=.137). There was also no significant difference between those who did not achieve the recommended Hb level and those who did in CRP values at the end of the study (1.5 compared to 0.9mg/dl, P=.223) and PTH (316.1 compared to 272.2pg/ml, P=.291). As expected, those without Hb within the target range at the end of the study received a higher mean dose of PTX than those who had it (914.3 compared to 763.6mg/day, P=.047).
DISCUSSION
Although there are publications on the use of PTX in renal disease in general, our case-control study demonstrates the benefit of using this drug for anaemia control in HD patients, which is observed over three months. PTX caused an increase of Hb in the short-term and a decrease in the ESA dose in the medium-long term, and as such, 61% of patients (some of them classified in our unit as resistant to ESA) achieved the recommended level of Hb. These effects were maintained over time and were achieved with an average PTX dose of 400mg/12 hours, which was well tolerated.
With regard to the effect of PTX on anaemia in renal patients, observational studies have been published with doses of 400 mg/day. One of them8 included 7 patients with an estimated glomerular filtration rate (eGFR) of less than 30 ml/min and another10 included 16 HD patients. Both reported an increase in Hb over six months. As for clinical trials, there is reference to the start of one in 2008 in Australia with plans to include 110 patients with an eGFR below 30ml/min, some on HD,13 but the results have not yet been published, and in another,11 published more recently, there were no differences in Hb and ESA dose after six months of treatment with 400mg/day of PTX compared to a placebo in 50 HD patients. In our case-control study, we observed an improvement in anaemia with PTX, although the initial and end mean doses were greater (approximately 800mg/day) than in the studies referred to. This could explain why the effect is observed already after three months, even for HD patients, in contrast to that described by the published assay.11 Perhaps a dose of 400mg/day may not be sufficient for some HD patients, although this should be confirmed in subsequent dose-finding studies.
In parallel with the increase in Hb, there was a significant decrease in the dose of ESA, making it comparable to that which the control group was receiving. In one of the aforementioned studies,8 in only 1 of the 14 patients who completed the study and with an increase in Hb was the ESA dose reduced. Given that it was a study of only four months of treatment, it is consistent with our results, since there was no reduction in the ESA dose either in our study at the third month, even when there was already an increase in Hb. The potential effect of PTX on ESA dose reduction became evident in the medium-long term (end of the study). The reduction observed in ESA, although the logical consequence of a sustained increase in Hb, is very interesting, not only because of the risks that high doses of ESA can have for certain conditions (high blood pressure and platelet dysfunction),14-17 but also due to the cost saving that avoiding these doses may involve, a matter of great importance in the current economic climate.
The improvement of anaemia with PTX has been related, among other mechanisms, to its anti-inflammatory properties.9,18,19 In fact, a recent study in 18 HD patients describes a decrease in inflammation parameters such as IL-6 and CRP after four months of treatment with PTX (400mg/day),7 and another, in CKD without dialysis describes decreases in the alpha tumour necrosis factor, CRP and fibrinogen after twelve months of treatment with doses of 800mg/day.20 In our study, we did not have data available for cytokine levels, but we did record CRP levels. The usefulness of CRP has been demonstrated in HD patients, not only as an inflammation marker, but as a potential mediator of reduced iron bioavailability and resistance to anaemia treatment.4 Although no significant decreases in CRP were observed in the treated group nor were there differences between groups, the consistency of the previous publications with regard to the impact of PTX on CRP7 levels and the numerical trend observed in our treated group (decrease in CRP), with a wide baseline variability (baseline SD of 4.8 in the group with PTX), leads us to suspect that this result is due to sample size limitations. With respect to ferritin, an indirect marker of inflammation that correlates positively with the level of IL-6 in one study,21 differences were noted between cases and controls, but no changes were observed with the treatment. In another study of patients with CKD who received PTX (800mg/day), there was also no significant decrease in ferritin.21 In our study, baseline ferritin levels that are higher in the treated group than in the control group can be considered as an indirect expression of a higher level of inflammation, and as with the abovementioned publication, they do not decrease with PTX, possibly due to the influence of other factors and the complex metabolism of ferritin.22
With regard to the potential role of other identifiable clinical factors3 of refractory anaemia in the treated group, it should be noted that at baseline they did not present greater comorbidity, measured by the Charlson index, vascular disease or poor control of hyperparathyroidism. In terms of nutritional status, although the dry weight and BMI were lower in the study group, they did not have a level of malnutrition. Neither were there any differences with regard to the cause of CKD and dialysis related factors,23 such as the number of patients with catheters (a factor associated with inflammation and anaemia), cases of conventional HD compared to on-line haemodiafiltration and the efficacy of HD.25 Even though in both groups the mean estimate of Kt as an adequate dialysis index was at the lower limit of normal for short sessions in some patients (advanced age, comorbidity and patient refusal of longer dialysis sessions), this situation did not prevent an improvement in Hb in patients treated with PTX. Only time on dialysis was much higher in the treated group and this factor may explain a certain negative impact on anaemia through mechanisms such as chronic inflammation.
Lastly, we refer to some of the drugs that patients received, such as ACEI and statins. Although a potential negative impact has been described on erythropoiesis associated with blocking angiotensin II, and in a publication it has been shown to be dose dependent,26 more recent studies do not display that effect.27 With regard to statins, a potential benefit has been described for anaemia control28 due to the decrease in prohepcidin levels (hepcidin precursor and inflammatory anaemia mediator).29 However, all our patients, treated and control, had low doses of ACEI and statins throughout the study, with no change in the dose and, therefore, the changes observed cannot be attributed to this drug.
With regard to the percentage of patients who did not achieve a recommended level of Hb (around 39%) at the end of study, the immunosuppressant treatment may play a role in the results, although a significant difference is not achieved (in this subgroup there were more cases with immunosuppressant treatment). This information could be considered for future studies as a predictor of poor response to PTX in the optimisation of anaemia control in HD or perhaps it could be considered that this subgroup of patients will respond to higher doses of PTX. Although the standard dose of this drug is 400mg three times a day and it does not seem that in renal failure there is a significant accumulation by taking it two or three times a day, more safety studies should be conducted in HD patients, especially for long-term treatments if higher doses are prescribed.
CONCLUSIONS
Although the study has limitations (single-centre, retrospective observational study with a limited n), we can argue that treatment with PTX at doses of 400mg/12 hours in HD patients may optimise anaemia control (increases Hb and reduces ESA doses), achieving approximately 60% response even in patients with a certain resistance to normal treatment. Furthermore, the effect observed is maintained over time, without tolerance or toxicity problems. These results should be confirmed in a randomised multicentre trial before including PTX routinely in the treatment of HD patients with no response to normal treatment and once the presence of treatable causes has been ruled out.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Table 1. Demographic and clinical characteristics of the treated and control groups
Table 2. Descriptive data of the study patients who were treated with immunosuppressants
Table 3. Haemoglobin levels, MIRCERA® dose, C-reactive protein, ferritin and intact parathyroid hormone by group
Figure 1. Distribution of the patients treated with pentoxifylline according to the haemoglobin range at the different stages