Atypical haemolytic uraemic syndrome (aHUS) is a thrombotic microangiopathy (TMA) mediated by uncommon and potentially fatal overaction of complement. The approval of eculizumab to treat aHUS has radically improved survival in this entity. The optimal duration of treatment in native kidneys remains the subject of debate.1,2
We present the case of a 26-year-old man, with no personal or family history of interest, who was transferred to our hospital due to acute oliguric renal failure requiring haemodialysis, severe anaemia and thrombocytopaenia. The blood test and blood smear performed were compatible with non-immune haemolytic anaemia.
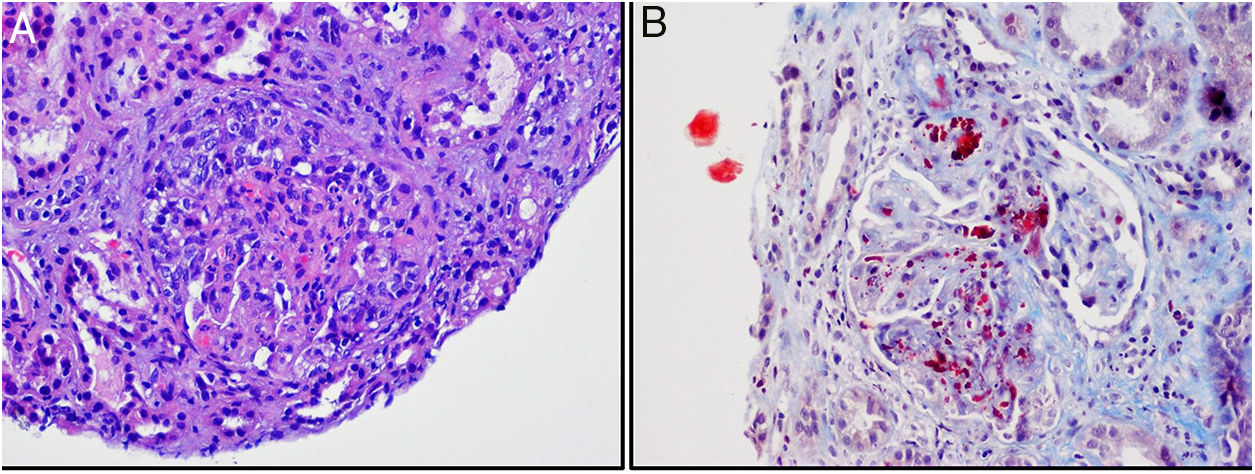
Given the suspicion of TMA, we analysed ADAMTS13 activity, which was normal. PCR for SHIGA toxin and stool culture were negative. After exclusion of other secondary causes of TMA, the diagnosis of aHUS was established. Genetic study and study of complement factors were requested. We started vaccination against meningococcus B and ACW135Y, and prophylaxis with ciprofloxacin. A renal biopsy was performed in which glomeruli were observed with thickening of capillary walls, segmental and focal fibrinoid necrosis, abundant schistocytes, and crescent formation in 3 glomeruli. The arteriole walls presented fibrinoid necrosis and schistocytes, as well as intraluminal thrombi (Fig. 1).
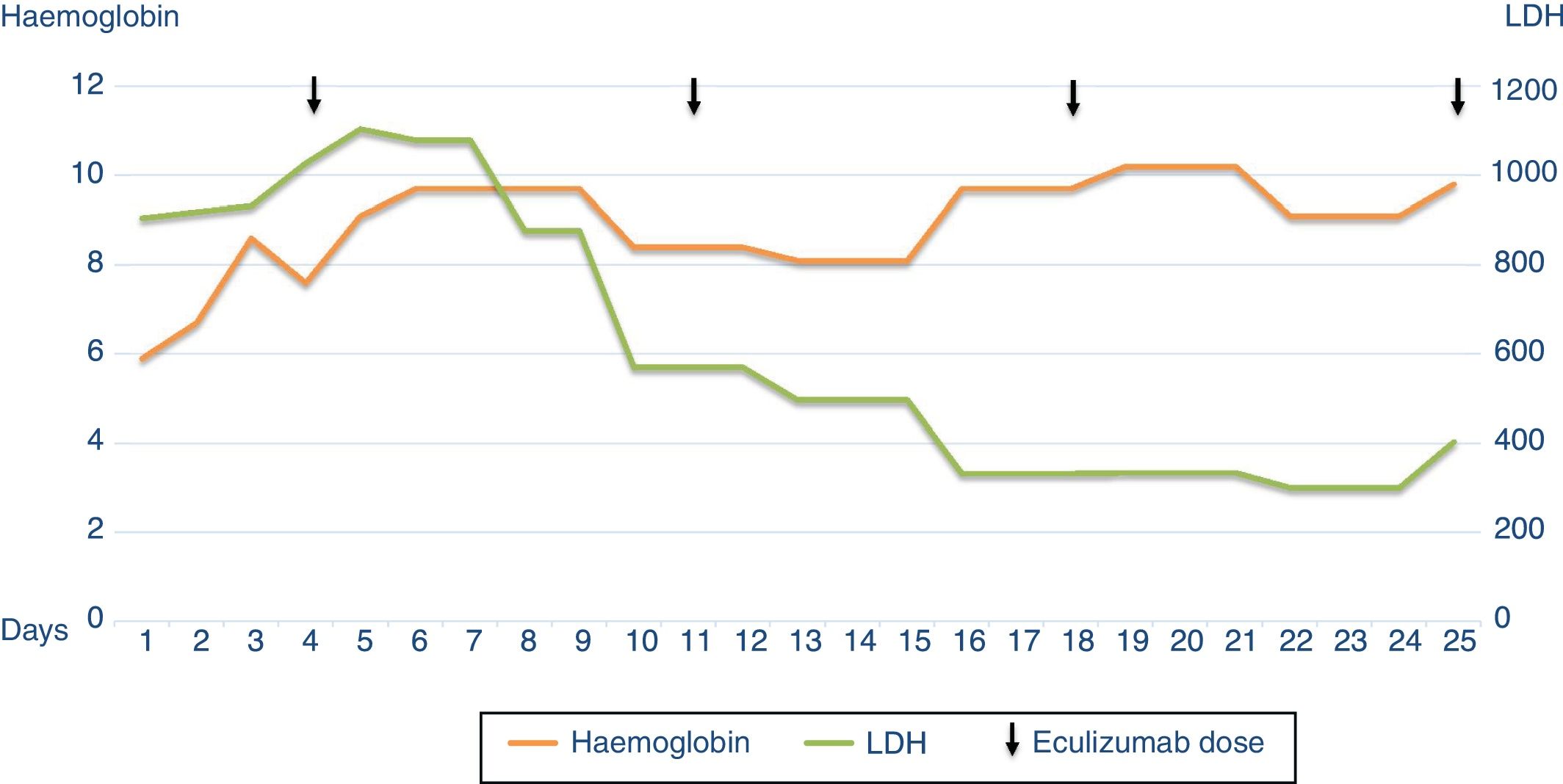
Four days after admission, treatment was started with eculizumab 900mg weekly for 1 month, showing good evolution with progressive normalisation of haematological parameters and, later, of renal function. After the second dose of eculizumab haemodialysis was no longer required, and the patient was discharged 21 days after starting treatment, with a creatinine level of 2.8mg/dl (Fig. 2).
Fifteen days after discharge, the patient presented with pneumonia and an episode of decompensated heart failure requiring hospitalisation, where dilated cardiomyopathy was detected, which responded well to medical treatment. In the year following discharge, the patient has continued with eculizumab and presents stable renal function with a creatinine level of 2–2.5mg/dl.
AHUS is a rare and serious disease with mainly renal involvement. Onset is generally abrupt, and it usually presents, as in our patient, with the triad of non-immune microangiopathic haemolytic anaemia, thrombocytopaenia and renal failure. Although renal involvement usually predominates, up to 20% of patients can present extrarenal manifestations, predominantly neurological, gastrointestinal and cardiac, due to the diffuse nature of the disease.3,4
The presence of complement gene mutations has been detected in approximately 40%–60% of patients with aHUS, and up to 10% have mutations in more than one gene.2 In our patient, polymorphisms associated with risk of aHUS were detected in the membrane cofactor protein (MCP) gene, together with deletion in CFH3-CFHR1, both in heterozygosis, as well as the presence of anti-factor H antibodies. These antibodies are present in 5%–10% of patients with aHUS, with consequences similar to those of mutations in the FH gene, and they seem to be related to the onset or recurrence of the disease.5 The presence of both polymorphisms, associated with the presence of anti-factor H antibodies, together with environmental factors that act as triggers, could justify the predisposition of this patient to develop aHUS (multiple hit theory).2,6
Genotypic–phenotypic characterisation of this syndrome has gradually improved thanks to the study of genetics and complement factors. Currently, one of the most debated issues is the duration of treatment with eculizumab.7 This decision usually depends on the patient's risk of relapse, taking into account various factors such as the patient's age, partial or total recovery of renal function, the presence of extrarenal manifestations, and the result of the genetic study.8
Various case series report that in 20%–30% of patients eculizumab was interrupted in order to avoid meningococcal infections, side effects of the treatment, and the high cost of the therapy.9 Approximately 20% of these patients present recurrence of TMA.5 It is crucial to monitor patients for early identification of recurrence and resumption of treatment. Despite the fact that most patients returned to their baseline status after eculizumab was resumed, it would be necessary to take into account the subclinical renal damage to which the patient is exposed with each relapse, and the subsequent progression of renal disease.10
Our patient showed significant haematological and renal involvement, and although he had already presented symptoms 2 weeks before admission, early start of treatment with eculizumab was key in his good evolution. Given this presentation, the persistence of chronic kidney disease and the subsequent development of extrarenal cardiac manifestations, we decided to continue with the treatment. More observational studies and a more complete collection of data on the interruption of treatment and its consequences would improve decision-making in this regard.
Please cite this article as: Naranjo Muñoz J, Garcia Garcia-Doncel A, Montero Escobar ME, Villanego Fernandez F, Millán Ortega I, Ceballos Guerrero M. Eculizumab en el síndrome hemolítico urémico atípico. ¿Hasta cuándo mantenerlo? Nefrologia. 2019;39:440–442.