La vuelta a diálisis tras fallo de trasplante renal (TX) es una situación cada vez más frecuente. En la vuelta a diálisis tras TX fallido suele darse una situación clínica similar o peor a la de los pacientes nuevos en hemodiálisis o diálisis peritoneal (DP). Aunque existen bastantes estudios sobre la situación clínica de los pacientes que vuelven a DP tras períodos largos con TX funcionante, no hay apenas información sobre la evolución de un subgrupo de pacientes que vuelven a DP tras fallo de TX a los pocos días o semanas de su realización. Objetivo: Evaluar si un corto período de tiempo con TX subóptimo y tratamientos/medidas agresivas pueden influir en la permeabilidad de membrana, la situación clínica y la eficacia dialítica al volver a DP. Pacientes y métodos: En 9 pacientes (53,5 ± 15,4 años, 5 hombres, 4 mujeres) procedentes de DP con fallo precoz de TX y vuelta a DP (25 ± 23 días, rango 10-64) de los cinco últimos años, se estudian datos analíticos de inflamación, nutrición, función renal, permeabilidad y eficacia de DP, en cuatro momentos de la evolución: previo al TX, inmediatamente a la vuelta a DP, al primer mes y al tercer mes de DP. Resultados: No se detectan diferencias significativas en la evolución de los parámetros de nutrición e inflamación. La diuresis desciende de forma significativa del volumen previo al trasplante al de la vuelta a DP y al primer mes en DP (p = 0,032), manteniéndose en niveles reducidos a los tres meses en DP. La UF se reduce de 1407 a 951 ml/día (p = 0,022) y de 314 a 260 ml/4 h (p = 0,018) en el test de equilibrio peritoneal al tercer mes en DP, sin cambios en el cociente dializado/plasma de creatinina. Kt/V y aclaramiento semanal de creatinina descienden ligeramente, manteniéndose en niveles adecuados de eficacia. Conclusiones: En esta pequeña muestra de pacientes que vuelven a DP tras fallo precoz de TX, no parece que las medidas que comporta el manejo de un injerto en riesgo en un corto espacio de tiempo afecten de forma importante a parámetros clínicos y de permeabilidad o eficacia peritoneal.
The return to dialysis after kidney transplant (TX) failure is increasingly common. On returning to dialysis after TX failure, there is usually a similar or worse clinical situation than in patients who are on haemodialysis or peritoneal dialysis (PD) for the first time. Although there are several studies on the clinical situation of patients who return to PD after long periods with a functioning TX, there is hardly any information on the progression of a patient subgroup returning to PD after TX failure a few days or weeks after transplantation. Objective: Assess whether a short period of time on suboptimal TX and aggressive treatment/measures may influence membrane permeability, the clinical situation and dialysis efficacy on returning to PD. Patients and method: In 9 patients (53.5±15.4 years of age, 5 males and 4 females) who had previously been on PD before early TX failure and had returned to PD (25±23 days, range 10-64) over the last five years, we studied laboratory data including inflammation, nutrition, kidney function, permeability and PD efficacy, at four points during progression: before TX, immediately after returning to PD and after one month and three months on PD. Results: We did not detect significant differences in the progression of nutrition and inflammation parameters. Diuresis decreased significantly from pre-TX volume to diuresis on return to PD and after one month on PD (p=.032), remaining at low levels after three months on PD. UF decreased from 1407 to 951ml/day (p=.022) and from 314 to 260ml/4h (p=.018) in the peritoneal equilibration test after three months on PD, without changes being observed in the creatinine dialysate/plasma ratio. Kt/V and weekly creatinine clearance decreased slightly and remained at adequate efficacy levels. Conclusions: In this small sample of patients, who returned to PD after early TX failure, it does not appear that the measures involved in managing a graft at risk over a short period of time have a major effect on clinical parameters and permeability or peritoneal efficacy.
INTRODUCTION
Kidney transplant (TX) failure with transfer to dialysis is an increasingly common situation, and as such, 20%-25% of patients on the TX waiting list have had TX failure.1 Some are treated with peritoneal dialysis (PD), and they are almost always patients who had been on PD before TX. However, most TX failure patients return to haemodialysis (HD) or begin HD even if they had previously been on PD, despite it being known that survival and complications in patients who are on PD or HD following kidney TX failure are similar.2-4 Most studies establish that survival in patients who are treated with PD after kidney TX failure is equal to that of new PD patients.2,5-8
Transfer to dialysis after kidney TX failure is usually marked by a similar or worse clinical situation than that of new HD or PD patients. There is a lot of information in the literature about resuming dialysis in kidney TX failure patients after quite a long period with a functioning renal graft, and much of the information highlights the poor clinical situation that many of these patients display due to the late initiation of dialysis. The poor clinical situation is based on worse kidney function (KF) in these patients compared to that of de novo dialysis patients,1,9,10 or in relation to associated comorbidity1,11,12 or laboratory data on dialysis after TX.1,9,13,14 These patients have a higher degree of anaemia, more systemic inflammation and more commonly have dyslipidaemia. The drug load, the significant loss of KF and the extended periods in TX clinics and not in pre-dialysis clinics may be some of the reasons for this situation.
There is little information on the clinical situation of patient subgroups in which TX failure occurs a few days or weeks after transplantation and there is a return to PD. We do not know if surgical aggression, the difficult postoperative situation in patients with a suboptimal graft and immunosuppression affect the peritoneal membrane, the efficacy of dialysis and the clinical situation on returning to PD within a short period of time. As such, the objective of the study was to assess whether a short time on suboptimal TX and aggressive treatments/measures could have an influence on membrane permeability, the clinical situation and dialysis efficacy on returning to PD.
PATIENTS AND METHODS
Out of the patients who had received a TX in the last five years in our Nephrology Department, we selected 9 PD patients who had experienced early TX failure (defined as failure within a few hours or days of transplantation) and who returned to PD a short time afterwards. Our patients included 5 males and 4 females with a mean age of 53.5±15.4 years (31-78), a time on PD previous to TX of 19.6±12.8 months (1.5-45.5) and time until PD after TX failure of 25±23 days (10-64). We considered 6 cases to be primary graft failure. The causes of TX failure were: 5 venous thromboses with no immunological cause (possible preservation failure), 1 thrombotic microangiopathy (preservation failure), 1 arterial thrombosis, 1 cortico-medullary necrosis (graft mycosis) and 1 of unknown cause. Immunosuppressive treatment varied in accordance with the type of graft received (brain death or asystole), with thymoglobulin, tacrolimus or basiliximab being used as induction drugs and with all patients receiving tacrolimus, mycophenolate mofetil and steroids to maintain immunosuppression.
We carried out blood test controls pre-TX, immediately after PD resumption, after a month on PD and after three months on PD. On each occasion, we studied nutrition data (albumin, pre-albumin, transferrin, total lymphocytes, protein catabolic rate [nPNA]), inflammation (high-sensitivity CRP [hs-CRP] and fibrinogen), KF with measurement of diuresis (24h), serum creatinine, creatinine clearance, glomerular filtration rate (GFR) and proteinuria. GFR was calculated with the Adequest (Baxter) software in conjunction with the kinetic study before kidney TX and in conjunction with the study carried out three months after returning to PD. Peritoneal permeability, creatinine dialysate/plasma (D/P) ratio with the peritoneal equilibration test (PET) and dialysis efficacy (weekly creatinine clearance, weekly Kt/V, ultrafiltration [UF] and peritoneal protein loss) were only measured in the study before TX and three months after returning to PD. UF was calculated by measuring 24h drainage collected in patients at the time of the scheduled revisions and with the UF obtained in the PET study with 2.3% glucose solution after 4h in the peritoneum. There were no episodes of peritoneal infection in the two months prior to TX, in the postsurgical period or in the post-TX study months. During the postsurgical period and after starting PD following kidney TX failure, no haemoperitoneum episodes were observed.
Statistical analysis
Patient characteristics were summarised using means and ranges (minimum and maximum). The Wilcoxon test was used to compare samples obtained before TX and after resuming PD, due to the distribution not being normal. All tests were carried out using the SPSS 15.0 statistical software (SPSS Inc. Chicago Ill. USA).
With regard to CRP, we should clarify that the distribution showed a wide range of values, and as such, it was converted using the Napierian logarithm. In all cases, a P value <.05 was considered to be statistically significant. We did not estimate the sample size for this pilot study. All analyses were carried out by comparing up to three months on PD against pre-TX.
RESULTS
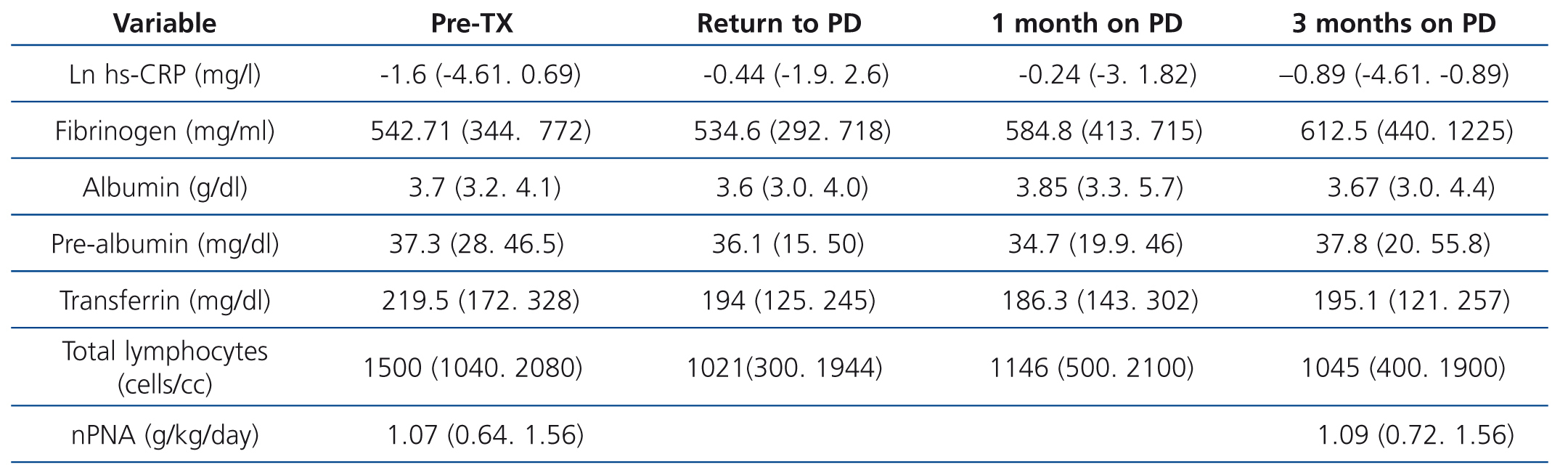
All patients resumed PD after kidney TX failure, but 5 required 4 to 9 HD sessions after TX failure. All patients underwent transplant nephrectomy. Inflammation and nutrition data pre-TX and after the three post-TX phases studied are displayed in Table 1. In the nutrition parameter progression, we observed that there were no differences in any of the parameters, except in the total lymphocyte count, which decreased on resuming PD and remained at levels below pre-TX levels during the three months of post-TX follow-up. nPNA measured pre-TX and three months after TX, in conjunction with the peritoneal kinetic study, did not change.
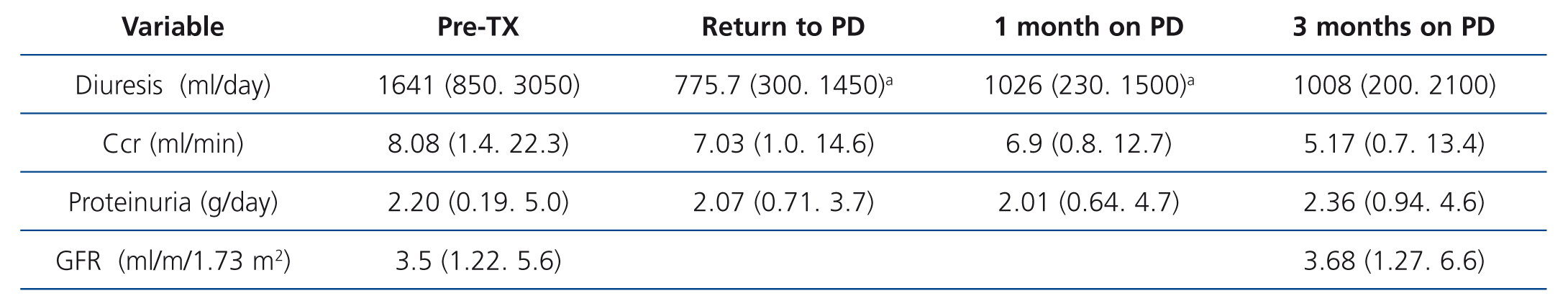
Diuresis decreased after TX (Table 2), with a significant volume decrease on PD resumption and in the first month after TX (P=.032), with low diuresis being maintained even after three months, which caused a non-significant reduction in creatinine clearance. One of the patients was anuric at the time of TX and 2 lost diuresis after TX. Proteinuria levels remained stable throughout progression.
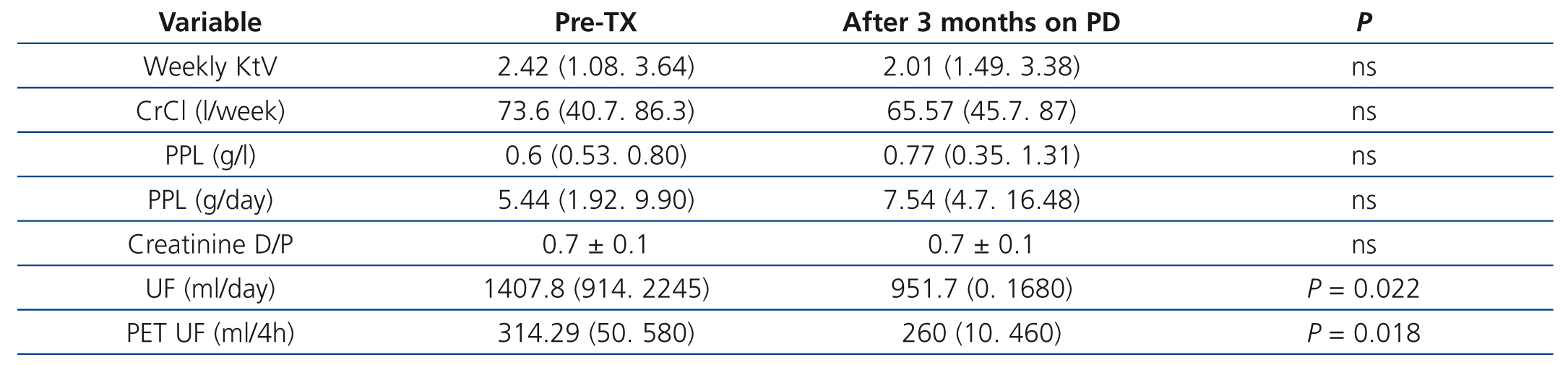
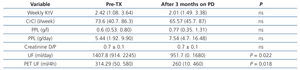
In the short period of time between the pre-TX peritoneal kinetic study (45±14 days) and that which was carried out three months after resuming PD after kidney graft failure, no differences were observed in the peritoneal membrane permeability or in dialysis efficacy (Table 3). The creatinine D/P ratio did not change after the short space of time between TX and the return to PD, with 57.1% high and medium-high transporters before TX and 62.5% after TX; in only one patient before TX and another in the tests carried out after three months on PD did we observe a creatinine D/P ratio of 0.80. Weekly urea Kt/V or total weekly clearance of creatinine decreased, but dialysis efficacy levels remained high. Peritoneal protein loss increased after patients resumed PD, but did not reach statistical significance. Only UF decreased significantly, from mean levels of 1407ml/day in the phase before TX to 951ml/day three months after PD was resumed (p=.022), and from 314ml/4h to 260ml/4h (p=.018) in the PET study.
DISCUSSION
The results of our study seem to indicate that patients who had been on PD before having a kidney TX for a short period and then suffered graft failure, did not have major changes in their clinical situation or in membrane permeability behaviour or dialysis efficacy. The small patient sample probably means that some trends observed could not reach statistical significance. A decrease in total lymphocytes was observed, which may be explained by immunosuppression induction with thymoglobulin, with major T lymphocyte-depleting effects.
It is definitely a short follow-up period, but the purpose of the study was really to discover whether the trauma suffered by the patient in order to save KF of a graft at risk, in some cases, and to keep the patient in a stable clinical condition after the trauma of major surgery, in others, could cause clinical or laboratory changes in the patient that would influence their return to PD.
Potential peritoneal membrane involvement in its permeability and efficacy does not seem unthinkable given the measures taken to save the postoperative situation, with major antibiotic therapy in some cases and aggressive immunosuppression in others, as well as systemic inflammation and malnutrition typical of prolonged hospitalisation etc.
It is important to know the peritoneal membrane condition of patients who are resuming PD after TX failure, because some authors have reported that the type of peritoneal permeability may influence patient and technique survival.15 We found very few references in the literature with respect to the behaviour of peritoneal permeability in patients on PD after TX failure. In some studies, such as that by Duman et al., there were no changes in membrane behaviour,6 and they observed no differences in the PET between new PD patients and those who had had a TX. Other authors observed an early increasing trend in the transportation of solutes in PD patients who had undergone kidney TX.2 It has also been reported that a high percentage of patients who start PD after TX behave as high transporters.16 Our results did not show changes in peritoneal permeability and there was only 1 patient before and one after TX who would be at the limit of what we consider to be high transporters. Only UF reduction experienced by our patients seems to indicate a moderate decrease in dialysis efficacy. Of interest is the reduction in diuresis, with a major decrease being observed in the post-TX period studied, which was maintained until three months after the return to PD. Although time on HD after TX in some patients was short, we cannot rule out its potential influence on diuresis reduction. The reduction of KF in dialysis patients who had a non-functional TX was higher than in patients who did not receive a transplant.17 Recently, the Peritoneal Dialysis Centre Group published results of PD patients who had received a TX, in which, despite considering it to be a good dialysis option after TX failure, worse clinical progression was observed with a rapid decrease in KF.18 None of the studies reviewed establish a design such as that which we introduced in our study, in which we analysed the changes that can occur after early failure and which, with the limitations mentioned, offers a new approach. Similar but more comprehensive studies with a greater number of patients should be carried out and they would probably require the collaboration of various hospitals with active TX units.
In conclusion, all our PD patients who had kidney TX failure resumed PD in a relatively short space of time, without significant changes being observed in general laboratory parameters or in peritoneal permeability and dialysis efficacy, which was maintained at adequate levels. Although no major differences were observed, except in the reduction of diuresis and UF between the pre-TX data and those observed when PD was resumed and in the trend found in different parameters in our study, a collaborative study on a higher number of patients is necessary.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Table 1. Progression of inflammation and nutrition parameters after kidney transplant failure and a return to peritoneal dialysis
Table 2. Progression of kidney function parameters after kidney transplant failure and a return to peritoneal dialysis
Table 3. Progression of dialysis efficacy and peritoneal permeability after kidney transplant failure and a return to peritoneal dialysis