INTRODUCTION
The diagnosis and treatment of anti-donor antibody-mediated rejection or humoral rejection (ABMR) is one of the main discussions at the moment in kidney transplantation. The search for histopathological markers that help us to diagnose ABMR has been more problematic, in contrast to the histological expression of cellular or tubulointerstitial rejection. Although the relationship between post-transplant anti-donor antibodies and the allograft’s prognosis has been a topic of discussion for a long time, led in the main by P.Terasaki,1,2 it was not until the beginning of 1990s when P. Halloran group3,4 studied the humoral mechanisms of rejection in greater depth. Feutch5 described the importance of C4d deposits as a marker that shows a humoral mechanism of allograft rejection in 1993. As a result of many studies carried out, the Banff consensus group established some diagnostic histopathological criteria of acute (ABMR) in 2003.6 These have been modified slightly in later meetings of the group. Furthermore, in 2005 this same working group looked at the physiopathological mechanisms causing chronic allograft failure in more detail and established the criteria defining chronic humoral rejection.7 In this review, we are trying to update any useful histopathological criteria for diagnosing acute and chronic ABMR.
ACUTE HUMORAL REJECTION
The distinction between acute (ABMR) and acute tubulointerstitial rejection or acute T-cell mediated rejection (TCMR) is important from an aetiopathogenic, clinical and therapeutic point of view, as acute ABMR has a worse prognosis and a higher percentage of cortico-resistance.8-10 TCMR has some histological criteria that are well-defined and standardised by international consensus, according to the Banff expert group;6,7,11-15 however, identifying acute ABMR in a renal biopsy is more problematic for three basic reasons. Firstly, there are no specific histopathological changes; secondly, the changes may not be indicative and conspicuous and, thirdly, it may coexist with TCMR.
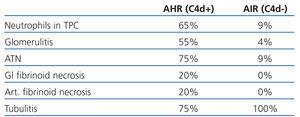
Halloran et al,3,4 at the beginning of 1990s, described three factors that defined a new entity different from traditional acute cellular rejection and hyperacute rejection: allograft dysfunction, neutrophils in peritubular capillaries and anti-donor HLA class I antibodies. Trpkov et al16 later described a series of histological markers, most significantly severe vasculitis which includes fibrinoid necrosis, glomerulitis, capillary thrombosis, necrosis and presence of polymorphonuclear neutrophils (PMN) in peritubular capillaries (PTC) that they defined as specific to antibody-mediated rejection, along with the presence of anti-donor HLA class I antibodies. More recent studies published by the Colvin group10,17 have confirmed and expanded these histological features defining acute ABMR (Table 1). It is worth mentioning the following histological marker: glomerular or arterial fibrinoid necrosis, as its presence is nearly pathognomonic in spite of its low incidence in the biopsy17 and, in addition, it indicates a worse prognosis of acute ABMR. Mauiyyedi et al found a 40% loss of the allograft in the first year in cases with fibrinoid necrosis compared to 27% in biopsies without necrosis.10
However, the histological diagnosis of acute ABMR is still problematic, especially in cases with few outward symptoms. The studies using immunofluorescence to detect immunoglobulins (IgG, IgM and IgA), complement factors (C3, C4 and C1q) and fibrin are not very accurate. Feucht et al5 were the first researchers to use anti-C4d antibody as a diagnostic test. This is a split product of complement factor C4, which is usually activated through the classical pathway. It forms a stable and long-lasting covalent bond with the permanent elements in the activation area. This is why it is useful as a diagnostic marker. These authors found that the one-year survival of the allograft was significantly lower in cases that expressed C4d (57% compared to 90%).5
Collins et al17 later found a correlation between the diffuse and intense C4d deposits (above 50%) in PTC, circulating anti-donor antibodies and histological findings indicative of acute ABMR (Table 1) and that the presence of neutrophils in PTC is more useful. Mauiyyedi et al10 showed a 95% sensibility and 96% specificity for C4d, as long as the presence of anti-donor antibodies was included as a diagnostic criterion of acute ABMR. However, there are other authors that have found a much lower sensibility (between 23% and 31%), but maintained a very high specificity (93%) for the presence of anti-donor anti-HLA antibodies.9,18 These discrepancies may be the result of the different sensitivities of the techniques used for detecting circulating anti-donor antibodies, the type of technique (Immunohistochemistry or immunofluorescence) and the type of antibody (monoclonal or polyclonal antibodies) used to detect the C4d . One could come to the conclusion with these findings, due to the high specificity, that a positive result indicates antibody-mediated rejection, as long as the deposits of C4d in PTC are diffuse and intense, but a negative result or focal staining does not rule it out.
Banff Criteria for acute humoral rejection
With all these previously reported data, the criteria for diagnosing acute ABMR or antibody-mediated rejection were established in the Seventh Banff Conference held in Aberdeen in June 2003,6,13 that included three essential aspects (Table 2):
1. Morphological evidence of acute tissue injury in the transplanted organ:
a. Acute tubular injury,
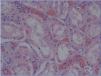
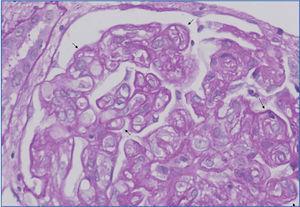
b. Neutrophils and/or mononuclear cells in peritubular capillaries and/or glomerulus, and/or capillary thrombosis (Figure 1),
c. Intimal arteritis/fibrinoid necrosis/intramural or transmural inflammation in the arteries.
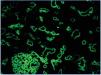
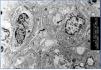
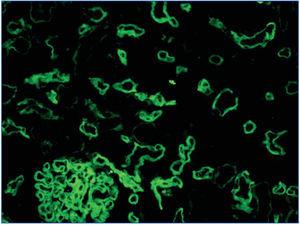
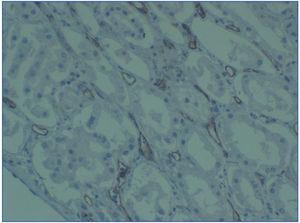
2. Immunopathological evidence of antibody activity: C4d and/or (rarely) immunoglobulins in peritubular capillaries. The C4d deposit must be diffuse and intense (>50%), see Figure 2 and 3.
3. Serological evidence of circulating antibodies against HLA antigens or other donor endothelial antigens.
These three criteria are needed to make a final diagnosis of acute ABMR. If there are indicative morphological criteria and positive C4d, but there is no proof of anti-donor antibodies, it must be classified as suspicion of acute ABMR.6 If suspicious histological symptoms are present and there is evidence of circulating antibodies, but there are no C4d deposits, it must be considered suspicious or consistent with acute ABMR.6
It is important to take into account that acute ABMR does not always appear in isolation and in fact, it may coexist with other conditions, making it difficult to diagnose. Acute ABMR is included in the second category of the Banff classification (antibodymediated rejection), but it may match three other categories (borderline rejection, acute cellular rejection or chronic allograft nephropathy),6 (see Table 2). Furthermore, there may be cases that have very little histological expression and only display acute tubular necrosis. C4d techniques are recommended in all cases of acute allograft dysfunction, even in cases that show no histological signs of humoral rejection.
Assessing C4d deposits
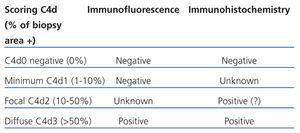
The C4d deposits in the peritubular capillaries must be diffuse and intense (>50%) to diagnose humoral rejection. A controversial aspect is what non-intense focal deposits of C4d mean. These focal deposits may mean that the antibody-mediated rejection is less intense, or even reflect a dynamic process. These deposits would correspond to an early phase of the process or a later phase where part of the deposits has already disappeared. Mendel,19 in a study carried out on protocol biopsies, revised the criteria used for C4d scoring. The author also observed that the focal deposits of C4d (deposits between 10% and 50% in peritubular capillaries) were associated with capillaritis and glomerulitis. Two recent studies found anti-donor antibodies in 38% and 69% of cases with focal deposits, respectively.15,20,21 Other authors suggested that cases with focal C4d have an intermediate prognosis between diffuse and negative cases.22-24 Criteria has been set to score C4d deposits based on these studies (Table 3).14,15
The conclusion can be reached that acute ABMR cannot be diagnosed based on a focal or mild deposit only, meaning that tests to detect anti-donor antibodies must be performed and that the patient must undergo a thorough follow-up.
The following points must also be assessed in the biopsy regarding C4d: C4d in the peritubular capillaries is more positive in the renal cortex than in the renal medulla; deposits of C4d in the arterioles, arterial intima and tubular basement membranes or glomeruli are not considered specific.
Methods for detecting C4d
There are two ways of detecting C4d: indirect immunofluorescence (Figure 2) and immunohistochemistry (Figure 3).
1. Indirect immunofluorescence (in two or three steps) using monoclonal anti-C4d antibody is a sensitive and reproducible method which is easy to interpret. However, it has a number of drawbacks: it does not work on formalin-fixed and paraffin-embedded samples. Therefore, it is necessary to use frozen samples and, furthermore, it is not permanent and deteriorates over time. In our experience, immunofluorescence is the gold standard method for detecting C4d.
2. The main advantage of immunohistochemistry using a polyclonal antibody over immunofluorescence is that it is carried out on paraffin-embedded samples. It is not necessary to use frozen samples. In contrast, it is a less reproducible technique and is harder to interpret, especially in focal or weaker cases. In our opinion, it must only be performed in cases when immunofluorescence is not possible, because it is not available or there is no frozen sample.
Studies by Mihatsch25 and Nadasdy,26 which compared both methods for analysing C4d, demonstrated that immunohistochemistry is not as sensitive as immunofluorescence, The Banff group created a basic description for interpreting the C4d results with both methods from these results (Table 4).14,15
Peritubular capillaritis
The presence and margination of inflammatory cells in peritubular capillaries is one of the histological markers defined in humoral rejection. Margination of polymorphonuclear neutrophils is the most accurate one, although the presence of neutrophils and mononuclear cells has been associated with C4d deposits in PTC.10,15,16 The Banff group, in its 2007 meeting,14 included a scoring system of peritubular capillaritis based on Gibson’s studies27 (Table 5). Several studies have confirmed the acceptability of this system and the association between high grades of capillaritis and antibody-mediated rejection.28,29 The grading must be carried out in the renal cortex, without assessing the medulla. Areas of pyelonephritis, areas next to infarcts and the vessels around aggregated lymphoid nodules must not be assessed either. The type of cells present must be stated (only mononuclear cells, minority or majority of neutrophils) and the extent of the capillaritis (> or <50%).
Inflammatory cells in humoral rejection
In addition to polymorphonuclear neutrophils and lymphocytes, other types of inflammatory cells may be present in acute ABMR. Magil and Tinckam,30 in a review of 23 biopsies with acute ABMR, and later on Colvin,31 found an association between the presence of monocytes/macrophages in the glomeruli and/or in the interstitial component and C4d deposits. This would suggest that it should also be included in the protocol of biopsy results. Other authors have shown that plasma cell-rich rejection is also associated with a mechanism of antibody-mediated rejection and with C4d deposits.32-36
C4d+ with no morphological evidence of active humoral rejection
The Banff classification14,15 recently included a new subcategory in acute ABMR: “C4d deposition without morphological evidence of active rejection”. This is defined as the presence of C4d deposits in peritubular capillaries and anti-donor antibodies, without histologic evidence of humoral/cellular rejection, with lack of glomerulitis, transplant glomerulitis, capillaritis, peritubular capillary lamination or acute tubular necrosis. If borderline inflammation (i1) is present, the diagnosis is inconclusive (Table 2).
The inclusion of this new category is based on studies carried out on protocol biopsies where C4d in CPT was found in 25%-80% of allograft biopses with normal histology incompatible transplantations. Evidence of acute ABMR was only seen in 4%-12% of cases.37,38 Other authors have found C4d deposits in 2%-26% of biopsies of allografts biopsies with normal histology.37,39 These incidental deposits of C4d do not necessarily mean that acute ABMR is present, according to the Banff experts.14
Hass,15,40,41 in studies carried out on biopsies of ABO-incompatible kidney transplants, quite often found C4d deposits without any histological signs of acute ABMR. He came to the conclusions that this may be an indicator of stable accommodation of the allograft, at least in ABO-incompatible transplants. The term accommodation is intentionally not used for this condition in the Banff classification, as the meaning of these finding and the long-term evolution of the allografts is unknown.
CHRONIC HUMORAL REJECTION
The diagnostic approach for chronic allograft failure (CAF) is complicated as there are many factors involved. These can be split into two groups: the immune factors (cellular or humoral) and the non-immune factors, including most importantly elderly donors, chronic ischemia, drug toxicity, hypertension, atherosclerosis and diabetes.42-45 It is important to distinguish between chronic rejection (CR), which must be limited to chronic allograft failure due to immunological causes, and chronic allograft dysfunction due to non-immunological causes for the prognosis and to offer a suitable treatment. This distinction is very often complex, as the histopathological features of the biopsies in this chronic period (interstitial fibrosis, tubular atrophy and glomerulosclerosis) are not very accurate and may be caused by immunological or non-immunological mechanisms. It is, therefore, important to determine the histopathological features that define CR and how they affect prognosis. The most characteristic histological findings of CR are chronic trasplant vasculopathy of the allograft, transplant glomerulopathy (TG) and peritubular capillary multilamination.46-55
TG was defined by Maryniak in 198549 as a heterogeneous glomerular disease consisting of double contours in the glomerular capillary walls (Figure 4) with special ultrastructural features, including electron-lucid subendothelial areas, glomerular basement membrane multilamination and mesangiolysis (Figure 5). An electron microscope must be used to diagnose it and the systematic use of this microscopy could enable TG to be diagnosed in very early stages. Studies carried out on protocol biopsies have shown that there are ultrastructural changes in TG in biopsies taken one month after the transplantation.15,56 The fact that it hides its aetiopathogenesis for some time, its similarity in the optical study and the electron microscope with thrombotic microangiopathy (TMA) led to demonstrating that the endothelial cell is the ‘target’ in both conditions. The endothelial cell would be the direct point of attack of the circulating anti-donor antibodies in TG. In both conditions, similar changes occur in the optical microscope as well as at the ultrastructural level, where they share comparable disorders, such as oedema or the subendothelial ‘rarefaction’ of the glomerular basement membrane, formation of double contours and even, ‘mesangiolysis’. There is increasing proof that TMA and TG are similar means of expression of the glomerulus in an endothelial lesion, although they have different clinical presentations, as there is no haemolytic-uraemic syndrome evidence in TG.50 In our experience, after reviewing 1110 biopsies of kidney transplantations, TG is the most common glomerulopathy with 42 cases. This represents 36.8% of glomerular diseases in the allograft and 3.7% of all allograft pathologies. It is important to highlight the poor prognosis of this condition, as 97% of cases lost the allograft in our experience.
Chronic graft vascular disease (CGVD) is still a principal histological marker and specific of chronic rejection and although its pathogenesis is debated, there seems to be signs of an antibody-mediated immunological mechanism. Histopathological features include a reduction in vessel lumen size caused by concentric myointimal proliferation which is usually accompanied by inflammatory cells in the acute phase. This is mainly made up of CD3-positive lymphocytes, macrophages and foamy histiocytes.52 This lesion progresses and becomes more sclerotic, and increased luminal occlusion and intimal signs of sclerosis are seen in this advanced stage. The histological key to differentiating CGVD from vascular disease secondary to atherosclerosis and/or hypertensive vascular disease is the existence of a concentric or segmental reduplication of the internal elastic lamina in cases of atherosclerosis and/or hypertensive vascular disease. This does not occur when the vascular lesion is the result of a CGVD, where the internal elastic lamina remains intact.53
Peritubular capillary multilamination (transplant capillary disease) (Figure 6) is another of the histological findings to diagnose of chronic rejection.54,55 Its diagnosis must be very accurate48 and requires an ultrastructural study. Monga et al46,47 described the association between TG and multilamination of the basement membrane of the peritubular capillaries (PTC). Drachenburg et al57 have shown that TG is associated with a severe multilamination of PTC (more than six laminates), while less serious levels of lamination (2-3 laminates) can also be seen in other types of glomerular diseases, both in the native kidney and in the allograft.
The role that the humoral mechanisms play in CR is currently under debate, although there is an increasing amount of evidence that it is involved in the pathophysiology of CAF. There are currently some theories suggesting that there are four successive phases in the evolution of antibody-mediation rejection, with CR being the final stage.58 Several studies have shown the association between histological or ultrastructural markers of CR and humoral mechanisms of rejection.59-63 Regele et al,59 in a review of 213 biopsies of transplants of over 12 months, found a statistically significant association between positive results for C4d and transplant glomerulopathy, lamination of the basement membrane of PTC and presence of mononuclear cells in PTC. Meanwhile, Mauyyedi et al60 found positive results for C4d in 61% of cases with CR compared to 2% in control cases. Furthermore, they found anti-HLA antibodies in 88% of the C4d+ cases, while no C4d- cases had antibodies.60
This association between C4d deposits, circulating anti-donor antibodies and histological findings of CR suggest that humoral mechanisms are involved in its aetiopathogenesis. These pieces of evidence led to a new category being included in the 2005 revision of the Banff classification7: Chronic or active late antibody-mediated rejection (Table 6). The term chronic or late indicates that is a slow, but active, process over a long time.7 The C4d deposits in PTC are evidence of an active humoral immunological process.
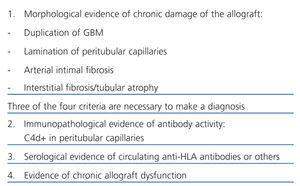
However, one must consider that, contrary to what was stated previously, there are several recent studies that have not found any correlation between TG and C4d deposits64-66 and some of these studies suggest that cell mechanisms or other mechanisms are involved in its development. Banu Sis et al15,67 showed that only 53% of TG with anti-HLA antibodies have C4d deposits, and suggested that this marker has a low sensibility in diagnosing chronic humoral rejection. For that reason, histological criteria, and C4d deposits and proof of anti-donor antibodies are needed for the final diagnosis of chronic antibody-mediated rejection, according to the Banff criteria. If there are morphological findings of CR and C4d deposits without any evidence of anti-donor antibodies, or antibodies without any evidence of C4d deposits, the diagnosis must be ‘suggestive of chronic antibody-mediated rejection’.7 It is important to specify in the biopsy whether there are histological signs that may be associated with late antibody-mediated rejection such as glomerulitis,68 aggregation of mononuclear cells in peritubular capillaries,59 interstitial inflammatory infiltrate and presence of plasma cells.24
In conclusion, mechanisms involved in the chronic pathology of the allograft are still unknown and not understood entirely and further studies will be needed that define better how humoral mechanisms are involved in its pathogenesis. However, there is sufficient evidence to state that the presence of transplant vascular disease, TG and capillary disease with multilamination of the basement membrane of PTC are useful histological markers for diagnosing CR. They are also indicators which imply an anti-donor antibody-mediated humoral rejection.
ADVANCES IN DIAGNOSING HUMORAL REJECTION
Recent studies, led in the main by the Halloran group, have introduced the possibility of carrying out molecular studies in the diagnosis of ABMR. Microarray studies have identified a set of endothelial genes expressed in ABMR which predict a poor prognosis.15,69 In addition, increased transcription of these endothelial genes is more sensitive than C4d at predicting graft loss, according to these authors, although it is less specific.15,69 They showed that in TG, a higher endothelial gene expression was indicative of a worse prognosis and an accelerated loss of the graft compared to cases of TG where there was no expression of these genes, regardless of the state of C4d.15,70
Endothelial gene expression in the biopsy of the graft may be useful as a sensitive and specific method for diagnosing ABMR, but the technique needs to be approved and standardised with new studies.15,69
CONCLUSIONS
1. Acute and chronic ABMR is currently one of the main problems of kidney transplantation.
2. The studies carried out in the last few years have helped identify a series of histological lesions suggestive of antibody-mediated rejection such as capillaritis, glomerulitis or fibrinoid necrosis for acute rejection, and transplant glomerulitis, chronic vascular disease and multilamination of the basement membrane of peritubular capillaries for chronic rejection.
3. The most important advance made in the diagnosis of ABMR was the discovery of the correlation between C4d deposits, degradation of complement factor C4 in peritubular capillaries and the presence of anti-donor antibodies. This has made this marker paramount for diagnosing ABMR.
4. The development of methods with increasing sensitivity has made it easier to identify anti-donor antibodies.
5. The ultrastructural study with an electron microscope is essential, especially in chronic lesions.
6. The Banff consensus group has established a set of diagnostic criteria for acute and chronic antibody-mediated rejection that include histological, immunopathological and serological criteria.
7. Endothelial gene expression in the biopsy of the allograft may be a useful method for diagnosing ABMR, but multicentre studies are needed to approve and standardise it.
8. Further studies would probably help in providing the definition of the diagnostic criteria of ABMR.
Table 1. Comparison of morphological findings between acute ABMR and ACR
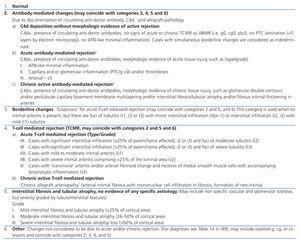
Table 2. Banff 97 diagnostic categories for renal allograft biopsies-Banff 09 update
Table 3. Scoring C4d deposits (percentage of biopsy or in five areas of a large increase)
Table 4. Scoring C4d in peritubular capillaries
Table 5. Criteria of peritubular capillaritis
Table 6. Diagnostic criteria of chronic active antibody-mediated rejection
Figure 1. Congestion and presence of inflammatory cells in peritubular capillaries
Figure 2. C4d deposits. Immunofluorescence
Figure 3. C4d deposits. Immunohistochemistry
Figure 4. Transplant glomerulopathy
Figure 5. Transplant glomerulopathy
Figure 6. Transplant capillary disease