Treatment of chronic renal disease in childhood must include assessment of social and psychological aspects involved in the perceived quality of life of the child and its family. Our objective has been to design a specific tool in Spanish for measuring quality of life in pediatric patients with chronic renal disease, since there is not a validated test for children at the moment. Results: We designed a specific questionnaire for renal disease in children based on the test of quality of life for adults with renal disease (KDQOL-SFTM) and on the test of quality of life for children with epilepsy (CAVE) adapting them to children with renal disease, denominating TECAVNER (Test of Quality of Life in Children with Renal Disease). Reliability of this questionnaire determined by alfa Cronbach coefficient was 0,92. Limitations: questionnaire determined by Test-retest reliability and construct validity were not conducted. In conclusion, this is a first approach for design a specific health related quality of life test in Spanish for children with chronic renal disease.
El tratamiento actual de la enfermedad renal crónica en la infancia debe incluir los aspectos sociales y psicológicos implicados en la calidad de vida del niño y de su familia. Nuestro objetivo ha sido desarrollar un instrumento de medida específico de la calidad de vida de los pacientes pediátricos con enfermedad renal crónica en español, ya que no existe actualmente ninguno validado para niños. Hemos desarrollado un cuestionario en español específico para enfermedad renal en niños basado en el test de calidad de vida para adultos con enfermedad renal (KDQOL-SFTM) y en el test de calidad de vida para niños con epilepsia (CAVE) adaptándolos a niños con enfermedad renal, al que denominamos TECAVNER (Test de Calidad de Vida en Niños con Enfermedad Renal). La fiabilidad de dicho cuestionario determinada por el coeficiente α Cronbach fue de 0,92. Las limitaciones del estudio consisten en que no se ha realizado validez de constructo ni test-retest. En conclusión, este trabajo constituye un primer intento para diseñar un cuestionario específico de calidad de vida relacionada con la salud en español para niños con enfermedad renal crónica.
INTRODUCTION
The difficulty in measuring health-related quality of life (HRQL) in children is due to the fact that there are specific instruments for some diseases and that the measuring instruments used in adults are not suitable for children. The health concept for children differs from that for adults, and factors such as employment or sexual activity are not applicable to the paediatric population.1 Independence is not applicable to children either, which constitutes an essential indicator of quality of life in adults, since children place more value on physical appearance or the number of friends they have. Another problem is that the information obtained from care givers does not always agree with the child’s view. Some authors suggest that a high concordance rate indicates poor quality of life in children, since childhood is characterised by the progressive achievement of autonomy and independence from the parents’ point of view.2-7 To receive information from the children themselves we need to develop specific questionnaires for this age, although for the very young children we will ask the closest caregivers.8 Perhaps the ideal solution would be to rely on both opinions. The most effective measurement methods in older children are self-applied questionnaires, while interviews are more suitable for younger children whose attention spans are limited.1 Questionnaires can be generic, regardless of the health-disease status, not created to detect differences between disease or therapeutic modalities and specific, which are applied to one conduct disorder and are suitable for evaluating the influence of different treatments. Generic measurements are used when the objective is to establish comparisons between different diagnostic or population groups; however, specific instruments need to be used to determine HRQL and to compare treatments or variables of a specific disorder. The questionnaires need to be reliable, valid, sensitive to change and adequately translated if the original is written in a different language,9 which explains why there are questionnaires for only a few paediatric diseases. Despite the fact that HRQL is being measured for many years in adults with chronic kidney disease (CKD), there is currently no Spanish-language questionnaire for children with CKD. We only have one English reference of a North American group of researchers working on its development.10 Our objective was to create a specific questionnaire on HRQL for children with chronic kidney disease (CKD) in Spanish. We wanted to determine the most important parameters associated with the disease and the questions that can be answered by children. We began with our previous study on the quality of life (QL) for children with CKD with a generic questionnaire,11 in which we found significant differences concerning the physical function, physical role and general health status healthy children.
MATERIAL AND METHODS
Before developing this specific questionnaire on HRQL for children with CKD, we assessed their QL and the most affected areas in relation to healthy group using the MOS-SF 20 generic questionnaire, completed by our patients with CKD and their parents, as well as a homogeneous sample of healthy children and their parents.11 At the same time, we searched the bibliography for specific questionnaires on the QL for children with chronic diseases9,12-14 and for adults with CKD, since there are no specific questionnaires for children with CKD in Spanish. KDQOLSFTM is one of the most used instruments in adults with CKD.15,16 It is a specific instrument for adults with renal failure, comprising thirty questions, some of which belong to the SF- 36 general health questionnaire of the IQOLA project, while others are specific to kidney disease. Among the specific questionnaires for children with chronic diseases we found four developed in Spain and another five that have been adapted.9 The questionnaire for asthmatic children (PAQLQ)17 and the quality of life scale for children suffering from cancer (ECVNO),18 both developed in 1996, as well as the quality of life questionnaire for children with spina bifida (1997)19 and CAVE,20 which measures the quality of life of children with epilepsy, stand out among the former. We decided to use CAVE as a basis for the creation of our questionnaire, since epilepsy, similar to CKD, is a chronic disease of long duration that generally requires frequent clinical and analytical tests, the taking of medication and a limitation of certain physical activities. From the KDQOL-SFTM questionnaire we kept the general health questions of the SF-36 that constitute the general perception of health and pain and for which we adjusted the scope of physical activity and emotional status to the actual characteristics of childhood according to our experience with children and the parents’ opinion. Concerning the specific questions on kidney disease, we kept those related to clinical symptoms and the aspects that mainly worry patients, which are the same in both children and adults, making small changes to adapt them to the paediatric age and substituting the questions related to the work environment and sexual activity with questions on school, playing, autonomy or family. Furthermore, we deleted the items referring to sleep, since sleep was not a concern for the children participating in our study nor was it mentioned by their parents. We added items referring to physical appearance and the number of hospital admissions and hospitalisation days, which influence children more than adults. From the CAVE questionnaire we kept the items on learning, autonomy, social relationships and school attendance, maintaining their initial wording, while we deleted the items on the intensity of the attack, its frequency and its management. From this questionnaire we did not use the item referring to the parents’ opinion when evaluating their opinion as widely as possible. The parents themselves completed the entire questionnaire separately from their children. The new questionnaire is multidimensional and covers the physical function and the psychological and social aspects recommended by the World Health Organisation, as well as other aspects. Once the first version of the questionnaire was completed, and after confirming that it was comprehensible for children over the age of nine and their parents, it was first given to children with kidney disease and their parents. This was done in order to determine the difficulties in its comprehension and completion. We therefore modified the wording of the most difficult-to-understand questions until the final version of questionnaire was completed, taking into account the opinion of parents and children. All of the patients and their parents participated voluntarily in the study, with previous verbal explanation of the contents of the questionnaire, and granted their consent. The questionnaire was given at the hospital when the follow-ups were carried out, and both patients and their parents were told how and who needed to answer each questionnaire. The questionnaires were completed in the waiting room of the examination room and were given back that same day. Seventy-one children with CKD took part: 33 with functioning kidney transplant, 11 on peritoneal dialysis, 5 on haemodialysis and 22 with conservative treatment. Children older than nine completed the questionnaire on their own while their parents did the same, trying to reflect how their children felt. For the children younger than nine the questionnaire was completed only by the parents. To determine the questionnaire’s reliability we carried out the Cronbach Alpha Coefficient, which studies the internal consistency of the different items that comprise the test. It calculates the correlation of the elements within the scale, quantifying the degree to which an instrument’s items and aspects measure the same concept. A coefficient greater than 0.7 indicates good internal consistency.
RESULTS
We developed a specific questionnaire on QL for children with CKD which we called TECAVNER (quality of life test in children with kidney disease), based on the CAVE20 and KDQOL-SFTM questionnaires.15-16 This questionnaire determines the general health status, physical status, pain and vital status and provides us with a particular view on the specific quality of life caused by kidney disease and how it is perceived by patients. The questionnaire is divided into 14 domains, some of which comprise various items or questions with a score ranging from 0 to 100 – a higher score indicating a better health status. The maximum score is 5,700, which corresponds to the best health status. The domains are as follows:
1. General health: personal evaluation of the current health status and resistance to the disease (2 items: questions 1 and 2).
2. Physical function or activity, that is, how the health status limits daily physical activities such as walking, playing, jumping, etc. (6 items: questions 3, 4.1, 4.2, 4.3, 4.4 and 4.5).
3. School attendance, that is, school absences caused by the disease (1 item: question 5).
4. Learning, that is, the cognitive field regarding other children of the same age (1 item: question 6).
5. Autonomy, that is, the different autonomy capabilities according to the child’s age, such as eating and getting dressed without help, going to school alone, etc. (1 item: question 7).
6. Social relationships, that is, how the health status interferes with daily social activities (1 item: question 8).
7. Pain, that is, its intensity and effects on everyday life (1 item: question 9).
8. Emotional well-being, that is, how emotional problems interfere with daily activities (6 items: questions 10.1, 10.2, 10.4, 10.5, 10.6 and 10.7).
9. Fatigue-energy, that is, the feeling of full energy, vigour or cheerfulness, and tiredness, fatigue or exhaustion (3 items: questions 10.3, 10.8 and 10.9).
10. Cognitive function (3 items: questions 10.10, 10.11 and 10.12).
11. Emotional presentation caused by kidney disease (4 items: questions 11.1, 11.2, 11.3 and 11.4).
12. Symptoms of kidney disease (9 items: questions 12.1, 12.2, 12.3, 12.4, 12.5, 12.6, 12.7, 12.8 and 12.9).
13. Effects of kidney disease on the patient’s life (14 items: questions 13.1, 13.2, 13.3, 13.4, 13.5, 13.6, 13.7, 13.8, 13.9, 13.10, 13.11, 13.12, 13.13 and 13.14).
14. Personal time dedicated to medical care (5 items: questions 14, 15, 16, 17 and 18).
The questionnaire’s reliability using the Cronbach Alpha Coefficient is 0.925 in the child self-report and 0.9156 in the parent proxy-report. Internal consistency is very good, since the Cronbach Alpha Coefficient is greater than 0.7 in all domains.
DISCUSSION AND CONCLUSIONS
This questionnaire (TECAVNER) can be used in children with CKD, is easy to answer and only requires approximately half an hour to complete. It is extremely reliable (Cronbach Alpha Coefficient at 0.925) with a high internal consistency in all domains, taking into account an acceptable reliability level if the coefficient is greater than 0.7. The questionnaire’s Spanish version for adults with kidney disease (KDQOL-SF) has an internal consistency with a Cronbach Alpha Coefficient for each domain between 0.67 and 0.87, while for the entire questionnaire the internal consistency is 0.93.21 The questionnaire’s limitations are that although its internal validity has been determined and is very good, we have not carried out the test-retest nor the construct validity which would absolutely validate the test. With this questionnaire we attempt to make the first step in the study of HRQL in children with CKD, and although we have yet to validate it, we have applied it to our group and the preliminary results obtained, included in this article,22 lead us to believe that it can be a good test for paediatric patients with CKD. The broadening of this study to a multi-centre one that includes a larger number of patients would grant greater reliability and validity to this questionnaire, since there is no other specific and validated instrument in Spanish in the current literature to measure the quality of life of paediatric patients with CKD. The results will help us understand the limitations of our patients and their concerns, thus we will be able to offer them a treatment according to their true needs. By including a higher number of patients we could determine the sensitivity to change and evaluate how the patients’ quality of life improves or deteriorates when changing one substitute treatment to another. Last but not least, we emphasised that the patients’ collaboration and that of their parents was, in general, excellent. Many of them, still without any significant problems detected, required and lacked psychological help both for the patients and for other family members who, although healthy, had a poor quality of life, anxiety and depression. The simple fact of completing the questionnaire made them feel better, since they could express their feelings and emotions, and they felt closer to the health professionals.
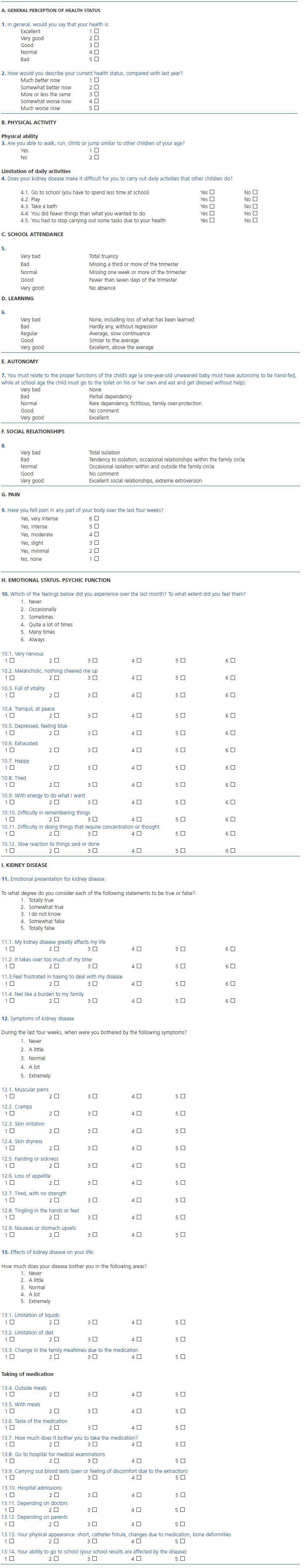
Annex 1. Specific quality of life test for children with chronic kidney disease (TECAVNER)
A. GENERAL PERCEPTION OF HEALTH STATUS
1. In general, would you say that your health is:
Excellent 1
Very good 2
Good 3
Normal 4
Bad 5
2. How would you describe your current health status, compared with last year?
Much better now 1
Somewhat better now 2
More or less the same 3
Somewhat worse now 4
Much worse now 5
B. PHYSICAL ACTIVITY
Physical ability
3. Are you able to walk, run, climb or jump similar to other children of your age?
Yes 1
No 2
Limitation of daily activities
4. Does your kidney disease make it difficult for you to carry out daily activities that other children do?
4.1. Go to school (you have to spend less time at school) Yes No
4.2. Play Yes No
4.3. Take a bath Yes No
4.4. You did fewer things than what you wanted to do Yes No
4.5. You had to stop carrying out some tasks due to your health Yes No
C. SCHOOL ATTENDANCE
5.
Very bad Total truancy
Bad Missing a third or more of the trimester
Normal Missing one week or more of the trimester
Good Fewer than seven days of the trimester
Very good No absence
D. LEARNING
6.
Very bad None, including loss of what has been learned
Bad Hardly any, without regression
Regular Average, slow continuance
Good Similar to the average
Very good Excellent, above the average
E. AUTONOMY
7. You must relate to the proper functions of the child’s age (a one-year-old unweaned baby must have autonomy to be hand-fed,
while at school age the child must go to the toilet on his or her own and eat and get dressed without help):
Very bad None
Bad Partial dependency
Normal Rare dependency, fictitious, family over-protection
Good No comment
Very good Excellent
F. SOCIAL RELATIONSHIPS
8.
Very bad Total isolation
Bad Tendency to isolation, occasional relationships within the family circle
Normal Occasional isolation within and outside the family circle
Good No comment
Very good Excellent social relationships, extreme extroversion
G. PAIN
9. Have you felt pain in any part of your body over the last four weeks?
Yes, very intense 6
Yes, intense 5
Yes, moderate 4
Yes, slight 3
Yes, minimal 2
No, none 1
H. EMOTIONAL STATUS. PSYCHIC FUNCTION
10. Which of the feelings below did you experience over the last month? To what extent did you feel them?
1. Never
2. Occasionally
3. Sometimes
4. Quite a lot of times
5. Many times
6. Always
10.1. Very nervous
1 2 3 4 5 6
10.2. Melancholic, nothing cheered me up
1 2 3 4 5 6
10.3. Full of vitality
1 2 3 4 5 6
10.4. Tranquil, at peace
1 2 3 4 5 6
10.5. Depressed, feeling blue
1 2 3 4 5 6
10.6. Exhausted
1 2 3 4 5 6
10.7. Happy
1 2 3 4 5 6
10.8. Tired
1 2 3 4 5 6
10.9. With energy to do what I want
1 2 3 4 5 6
10.10. Difficulty in remembering things
1 2 3 4 5 6
10.11. Difficulty in doing things that require concentration or thought
1 2 3 4 5 6
10.12. Slow reaction to things said or done
1 2 3 4 5 6
I. KIDNEY DISEASE
11. Emotional presentation for kidney disease:
To what degree do you consider each of the following statements to be true or false?:
1. Totally true
2. Somewhat true
3. I do not know
4. Somewhat false
5. Totally false
11.1. My kidney disease greatly affects my life
1 2 3 4 5 6
11.2. It takes over too much of my time
1 2 3 4 5 6
11.3.Feel frustrated in having to deal with my disease
1 2 3 4 5 6
11.4. feel like a burden to my family
1 2 3 4 5 6
12. Symptoms of kidney disease
During the last four weeks, when were you bothered by the following symptoms?
1. Never
2. A little
3. Normal
4. A lot
5. Extremely
12.1. Muscular pains
1 2 3 4 5
12.2. Cramps
1 2 3 4 5
12.3. Skin irritation
1 2 3 4 5
12.4. Skin dryness
1 2 3 4 5
12.5. Fainting or sickness
1 2 3 4 5
12.6. Loss of appetite
1 2 3 4 5
12.7. Tired, with no strength
1 2 3 4 5
12.8. Tingling in the hands or feet
1 2 3 4 5
12.9. Nauseas or stomach upsets
1 2 3 4 5
13. Effects of kidney disease on your life:
How much does your disease bother you in the following areas?
1. Never
2. A little
3. Normal
4. A lot
5. Extremely
13.1. Limitation of liquids
1 2 3 4 5
13.2. Limitation of diet
1 2 3 4 5
13.3. Change in the family mealtimes due to the medication
1 2 3 4 5
Taking of medication
13.4. Outside meals
1 2 3 4 5
13.5. With meals
1 2 3 4 5
13.6. Taste of the medication
1 2 3 4 5
13.7. How much does it bother you to take the medication?
1 2 3 4 5
13.8. Go to hospital for medical examinations
1 2 3 4 5
13.9. Carrying out blood tests (pain or feeling of discomfort due to the extraction)
1 2 3 4 5
13.10. Hospital admissions
1 2 3 4 5
13.11. Depending on doctors
1 2 3 4 5
13.12. Depending on parents
1 2 3 4 5
13.13. Your physical appearance: short, catheter fistula, changes due to medication, bone deformities
1 2 3 4 5
13.14. Your ability to go to school (your school results are affected by the disease)
1 2 3 4 5
J. TIME USED IN KIDNEY DISEASE
If you received medical care in the last four weeks, can you remember how many times you required seeing the doctor or be hospitalised?
14. How many nights did you spent in hospital?
0 days 1-2 days 3-5 days 6-10 days 11-15 days 16-20 days >20 days
15. How many times did you go to hospital for an examination or to the emergency department?
0 days 1-2 days 3-5 days 6-10 days 11-15 days 16-20 days >20 days
16. How many times did you require a visit by a nurse or another health professional at home?
0 days 1-2 days 3-5 days 6-10 days 11-15 days 16-20 days >20 days
17. How many times did you require general care from your family members (e.g. to take a bath, get dressed, etc.)?
0 days 1-2 days 3-5 days 6-10 days 11-15 days 16-20 days >20 days
18. How many times did you call the hospital, your doctor or nurse for a medical examination over the telephone?
0 days 1-2 days 3-5 days 6-10 days 11-15 days 16-20 days >20 days
SUPPORTING SUMMARY
1. What is known of the issue?
There are generic questionnaires that measure the quality of life in children, allowing for a comparison to be made with healthy groups; however, there are no specific questionnaires on the quality of life of children with kidney disease that allow for the assessment of variations within the disease itself. Over the last years, four questionnaires have been developed in Spanish and another five have been adapted, which are specific on the quality of life of children with certain chronic diseases (reference 19), but none of which are on kidney disease. In the United States a group of researchers (reference 20) is working on the development of a specific questionnaire for children with kidney disease in English, which should be translated into Spanish and validated prior to its application.
2. What contribution does the study make?
Our study contributes to the creation of a specific questionnaire that measures the quality of life of children with kidney disease in Spanish, with a reliability rate of 0.92 that is determined by using the Cronbach Alpha Coefficient with an internal consistency greater than 0.7 (very good) in all aspects. It is the only Spanish-language questionnaire for children with kidney disease, and we only know of one other questionnaire, which is being developed in English.
Specific quality of life test for children with chronic kidney disease (TECAVNER)