To the Editor:
In an attempt to satisfy the growing demand for kidney grafts, several hospitals have gradually expanded their criteria for accepting marginal and sub-optimal kidneys (generally derived from elderly donors or those with a risk for a potential reduction in nephron mass), which currently constitute 50% of all grafts.1 These organs are associated with a greater incidence of acute ischaemic renal failure, acute tubular necrosis (ATN), greater plasma creatinine levels, and delayed graft function, which can all contribute to increasing the rates of acute rejection.2
Calcineurin inhibitors (cyclosporine A and tacrolimus) are essential medications for maintenance immunosuppression therapy. The mechanism of action of these drugs is to block interleukin (IL) 2, IL-2 and IL-4 receptors, and gamma interferon. The most characteristic side effect of these drugs is nephrotoxicity, due to the increased expression of transforming growth factor beta (TGF-ß), which contributes to interstitial fibrosis and the synthesis of nitric oxide and endothelin, which have vasomotor properties.3 This effect is increased in marginal kidneys, which often present interstitial fibrosis, vascular involvement, and glomerulosclerosis.4
In order to minimise the nephrotoxicity of these drugs in sub-optimal grafts, several different strategies have been tested, such as reducing doses or delaying introduction of treatment. The latter has produced positive long-term results, with low rates of acute rejection and acceptable levels of renal function.5
We examined the short-term influence of delayed introduction of calcineurin inhibitors among patients who received kidney transplants from marginal donors at the University Hospital of Salamanca, comparing the effects of the drug in these patients with another group of recipients of young kidney transplants who received treatment from the start. We performed an observational, descriptive, cohort study of all transplants carried out at our hospital between 2008 and 2011. Patients were divided into two groups: recipients of standard kidneys, who received tacrolimus treatment starting before the transplant was performed, and sub-optimal kidney recipients (donor and recipient older than 55 years of age, cold ischaemia time greater than 1 day, cardiovascular death, serum creatinine greater than 2mg/dl, and non-heart beating donors), in which the medication was introduced on the fourth day. All other immunosuppression was maintained in both groups: basiliximab, steroids, and mycophenolate mofetil, as well as anti-infection treatment.
The variables analysed included: creatinine at the time of patient discharge and the presence of ATN, acute rejection, and infections within three months after transplantation. We used SPSS® statistical software, version 15.0, for all statistical analyses, which involved Student’s t-tests and chi-square tests, using a significance level of P<.05 and expressing variables as percentages, means, standard deviation, and relative risk.
During the study period, a total of 160 patients received kidney transplants. Of these, 43.8% received pre-transplant tacrolimus, and 56.3% received tacrolimus starting on the fourth day post-transplant.
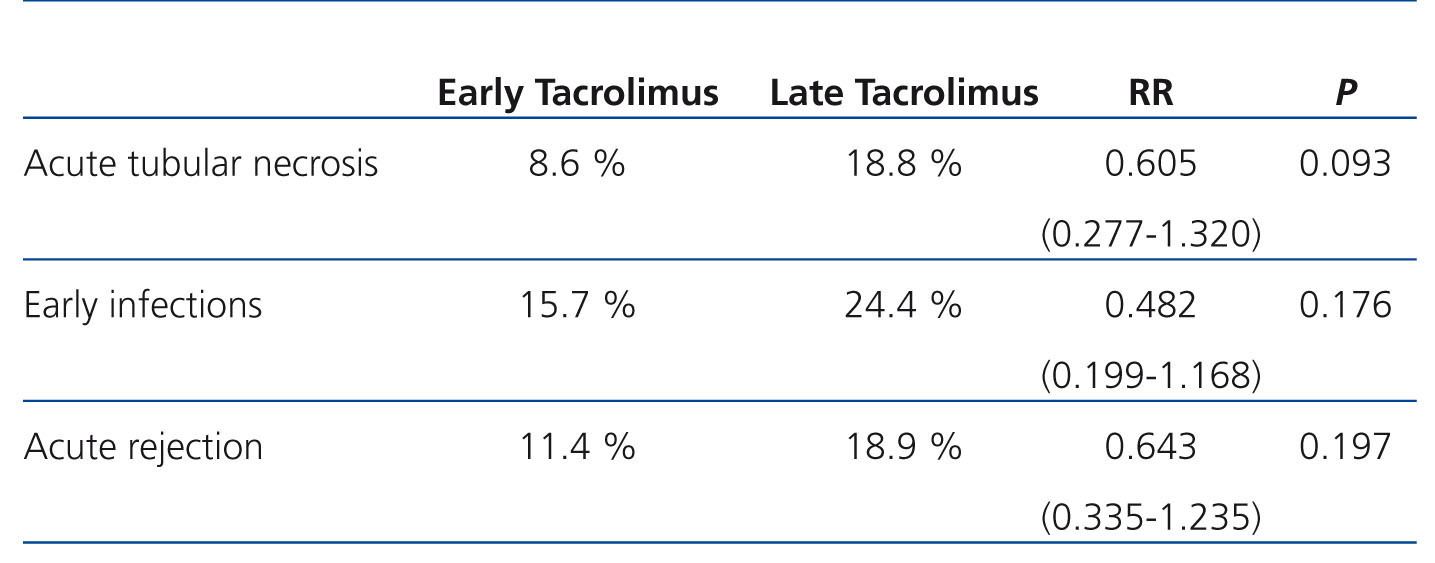
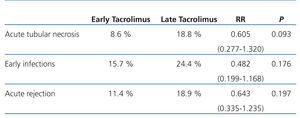
Mean creatinine in the early introduction group was 1.9+1.25mg/dl, and this value was 2.64+1.48mg/dl in the late introduction group (P=.098). All other results are expressed in Table 1.
The late introduction of calcineurin inhibitors is safe in the short-term, since, in comparison with grafts from young donors in which recipients received immunosuppression from the start, these patients did not exhibit a significant increase in parameters for renal dysfunction or secondary side effects. In this manner, the differences observed between the two groups may be due simply to the worse condition of marginal kidneys, rather than being due to any negative influence of the late introduction of medication on the immediate post-transplant patient evolution.
The results of our study, together with the results from previous studies that have established long-term safety, support the use of this immunosuppression regimen for treating recipients of marginal kidney grafts.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Statements collected
This study has had no source of sponsorship.
Table 1. Comparison of the appearance of complications between the two study groups