Objetivo: Estudio retrospectivo observacional multicéntrico de los pacientes trasplantados renales pediátricos, para conocer la situación actual frente al citomegalovirus (CMV), antes de participar en un ensayo clínico internacional de profilaxis durante 6 meses. Material y métodos: Se incluyen 239 pacientes menores de 19 años, procedentes de 5 centros entre 2005-2009, con seguimiento de 1 año. Resultados: La serología frente al CMV era negativa en 54 % de los receptores y 34,7 % de los donantes. Sesenta pacientes (25,1 %) fueron considerados de alto riesgo [Donante (D)+/Receptor (R)-] para infección por CMV. El 80,8 % realizó algún tipo de profilaxis, incluyendo todos los pacientes de alto riesgo, un tiempo medio de 65,5 días. La incidencia de positivización de CMV fue del 24,26 % (58 pacientes de los 239 trasplantados), con una incidencia de enfermedad del 6,7 %. La infección por CMV se asociaba con el estatus serológico (D/R) (p < 0,001), con la seropositividad del donante (p < 0,001) y con un tiempo de profilaxis < 20 días (p < 0,05). No hubo ningún caso de éxitus o pérdida del injerto secundaria a la infección, ni de resistencia al tratamiento. Conclusiones: La principal estrategia preventiva frente al CMV en el trasplante renal pediátrico en nuestro país es la quimioprofilaxis (81 %), con una incidencia de CMV del 24 % y de enfermedad del 6,7%, sin graves efectos directos ni indirectos en el primer año postrasplante. Su incidencia está relacionada, fundamentalmente, con el estatus serológico D/R y con la seropositividad del donante.
Objective: An observational retrospective multicentre study of kidney transplants in paediatric patients was performed to evaluate the current situation of cytomegalovirus (CMV) in this population, before our participation in an international clinical trial of prophylaxis for 6 months. Material and method: Our study included 239 patients aged <19 years, from 5 Spanish centres between 2005-2009, with 1 year of follow-up. Results: Pretransplant CMV serology was negative in 54% of recipients and 34.7% of donors. Sixty patients (25.1%) were considered at high risk (D+/R-) for CMV infection. Prophylaxis was used in 80.8% of recipients, including all high-risk patients, for an average time of 65.5 days. CMV viraemia occurred in 24.26% (58 cases among 239 patients), and disease in 6.7%. CMV infection was associated with serological status (D/R) (P<.001), positive serology of the donor (P<.001) and duration of prophylaxis <20 days (P<.05). There were no cases of patient or graft loss secondary to infection, nor resistance to treatment. Conclusions: The main preventative strategy against CMV in paediatric renal transplantation in our country is chemical prophylaxis (81%), with an incidence of infection and disease of 24% and 6.7%, respectively. There were no serious direct or indirect effects in the first year post-transplant. The incidence is mainly linked with serological D/R and positive donor status.
INTRODUCTION AND OBJECTIVE
Cytomegalovirus (CMV) continues to represent one of the most important opportunistic pathogens due to its frequency in kidney transplant patients, causing both direct complications (acute viral syndrome, invasive syndrome) and indirect complications (opportunistic infections, graft rejection, cardiovascular damage, etc.).1,2 Currently, two different strategies are commonly used to prevent this disease in adult patients: prophylaxis, which is administered during the first 3-6 months following transplant primarily in high-risk patients (donor+/recipient – [D+/R-]), or preemptive therapy, which is commenced as soon as viraemia is detected through periodical laboratory tests. Prolonging prophylactic treatment to 6 months appears to reduce the incidence of late onset disease,3 although there is no clear consensus regarding its use due to the development of possible anti-viral resistance, drug toxicity, reduced compliance, and increased cost, among others.
Although adult and paediatric patients share similar risk factors for developing post-transplant CMV disease, the paediatric patient has a higher frequency of high-risk patients given the greater proportion of recipients with negative CMV serology to seropositive donors, and as such, a greater probability of primary viral infection.4,5 Despite this situation, very few studies have been carried out in children, and management strategies are for the most part based on results from the adult population.
In general, the most commonly used strategy in modern paediatric kidney transplants is a mix of the two strategies proposed for adult recipients: a relatively short prophylactic period followed by monitoring viral load in order to provide preemptive treatment in the case of positive viraemia.5,6 Although several studies have reported a reduction in the prevalence of CMV disease to only 4% in one cohort of paediatric recipients of kidney transplants, including a 50% reduction in high-risk patients as compared to those who did not receive prophylaxis,7 multi-centre trials with a prospective and randomised study design are needed to firmly establish which strategy to follow, the appropriate duration of prophylactic treatment, and/or monitoring for preventing disease in these patients.
After committing to participate in a trial of these characteristics, we decided to first compile information regarding the situation of kidney transplants in paediatric patients in Spain, what proportion of these are high risk, the incidence of disease/infection by CMV, and the current state of clinical practice regarding prophylaxis/preemptive therapy.
MATERIAL AND METHOD
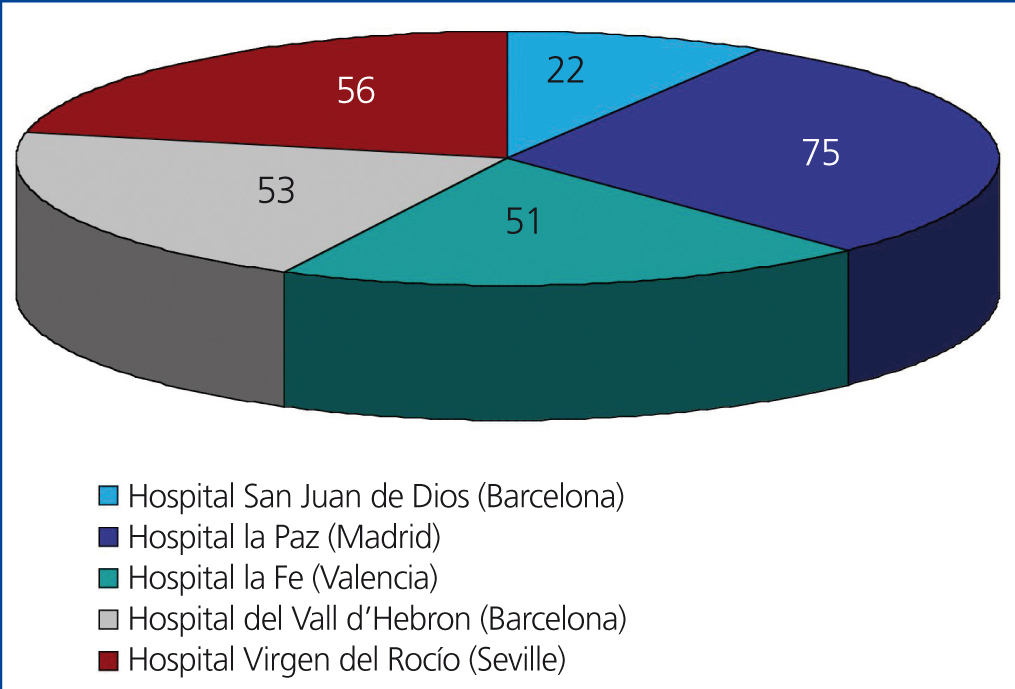
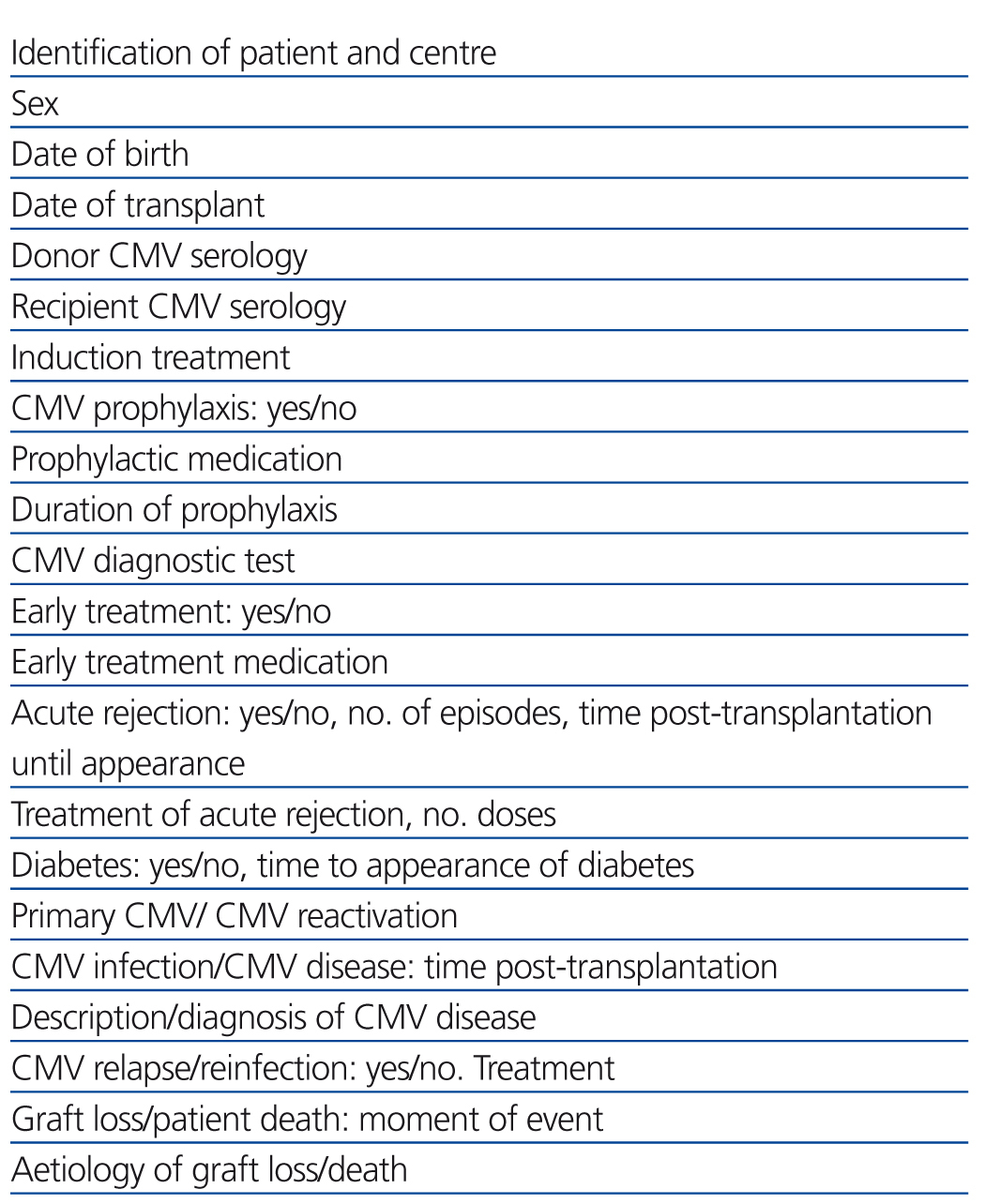
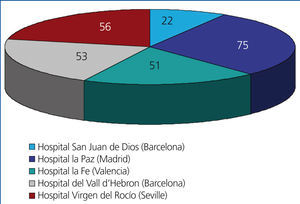
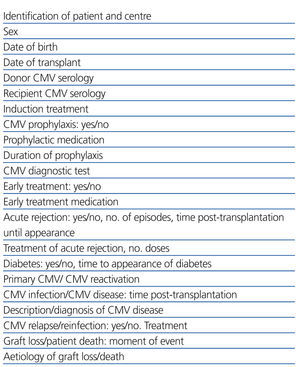
We performed a retrospective observational study using data extracted from clinical histories and databases at each of the different participating hospitals, referencing the variables summarised in Table 1. This involved 5 of the 7 paediatric nephrology units where virtually all paediatric kidney transplants in Spain are performed, with the distribution displayed in Figure 1. The studied population included all paediatric patients who received transplants at these 5 centres during a 5-year study period (1 January 2005 to 31 December 2009). We compiled data from each of these patients collected during the first year following transplantation. We included all patients in our study that surpassed 6 months with a functioning renal graft.
Definitions: Infection was defined as a detection of antigenaemia (pp65 CMV in leukocytes) or a positive polymerase chain reaction (PCR) test for CMV, and we considered disease to be present when the virus detection was supplemented by viral syndrome (fever >38º not produced by other causes, with at least leukopenia) with or without accompanying symptoms of specific organ involvement, and requiring anti-CMV treatment.
We differentiated between primary CMV, in patients who, prior to transplantation, had negative serology test results for CMV, and reactivation of CMV, when prior serology tests were positive, with no differentiation between reactivation and reinfection, since the strain of CMV was not taken into account. We considered recurrent infection to be a new detection of CMV at least 4 weeks after having controlled the first infection.
We defined chemical prophylaxis as the use of an anti-CMV agent in the absence of evidence of active infection as a means to prevent disease acquisition. We defined preemptive treatment as the use of anti-CMV medication early, in patients with asymptomatic replication of virus detected through periodical monitoring using PCR or antigenaemia tests. The defined value for positive replication was dependent on the experience at each institution, and was not evaluated in our study.
In patients who received prophylaxis with valganciclovir, doses were adjusted to body surface area and renal function.8
Statistical analyses were carried out using IBM SSPS® Statistic software, version 19.0: for descriptive analyses of qualitative study variables, we used absolute and relative frequencies and compared study groups using chi-square or Fischer’s exact tests as appropriate. The variable “age” did not follow a normal distribution, and so we summarised values as median [P25-P75] (interquartile range), and compared medians using the non-parametric Mann-Whitney U test. The level of statistical significance was set at P<.05.
RESULTS
We compiled the results from 257 patients who had received renal transplants during the study period, with no distinction made between living or cadaveric donor. Patient age ranged between 6 months and 19 years, and 160 patients were males, 97 females.
Due to various reasons, 18 of these patients were excluded from the final analysis:
- Eight were excluded due to early graft loss, all of which occurred during the first 72 hours either due to thrombosis (n=7) or vascular complications related to the surgical intervention (intra-operative loss due to vascular complication with uncontrollable bleeding).
The remaining 10 patients were excluded from our study due to an insufficiently long follow-up period. The reasons for this were: mycotic pseudoaneurysm in one patient necessitating a transplantectomy one month after transplantation; 5 cases of graft rejection (one case of hyperacute rejection, one case of acute rejection in a hyper-immunised patient four days after transplantation, one case of kidney rupture one week after transplantation due to severe cellular rejection, and two cases of acute humoral rejection due to major histocompatibility complex class I antigen A antibodies (anti-MHCA) after 10 and 15 days, respectively); 3 patients died due to infections (one due to septic shock of an unknown cause 19 days after transplantation, one due to infection after 3 months in the form of cerebral aspergillosis, and one due to multi-organ failure following an adenovirus infection 5.5 months after liver and kidney transplant); the last case excluded was due to a loss of follow-up after 5 months when the patient left the country.
Our analysis involved the remaining 239 patients, which were composed of 147 males and 92 females and had a median age of 11.92 years (range: 0.5-19 years), with a standard deviation of 5.3 years.
In four of these patients, both kidney and liver transplants were performed.
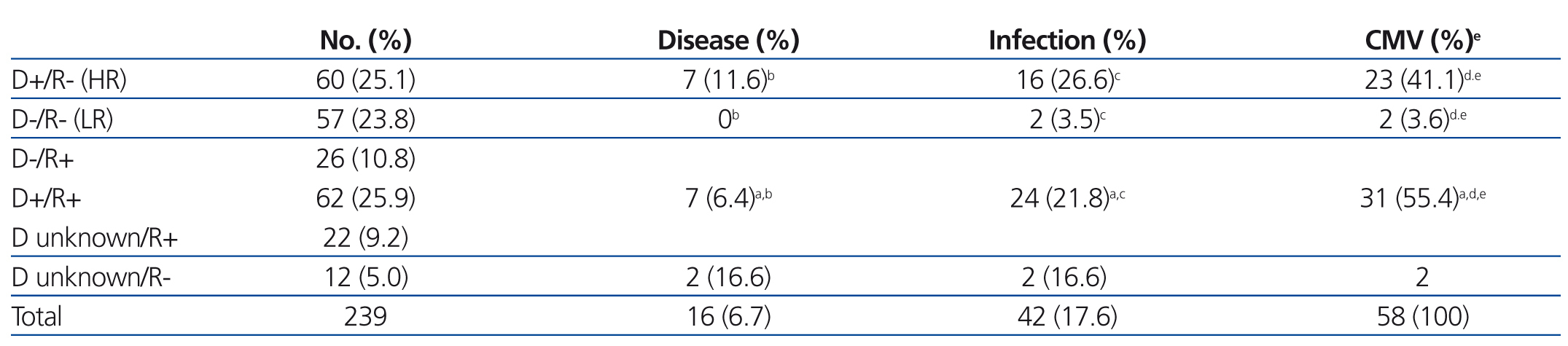
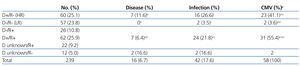
The CMV status for the study patients was the following: 129 recipients (54%) and 83 donors (34.7%) had negative serological test results for CMV, and 110 recipients (46.%) and 122 donors (51%) were seropositive, with the status of the remaining 34 donors (14.2%) unknown. In terms of donor/recipient (D/R) pairs, 60 of these (25.1%) were considered to be at a high risk of CMV infection due to a situation of D+/R-, and 57 pairs (23.8%) were not considered to be at risk due to D-/R- status (Table 2).
CMV management was different at each different hospital:
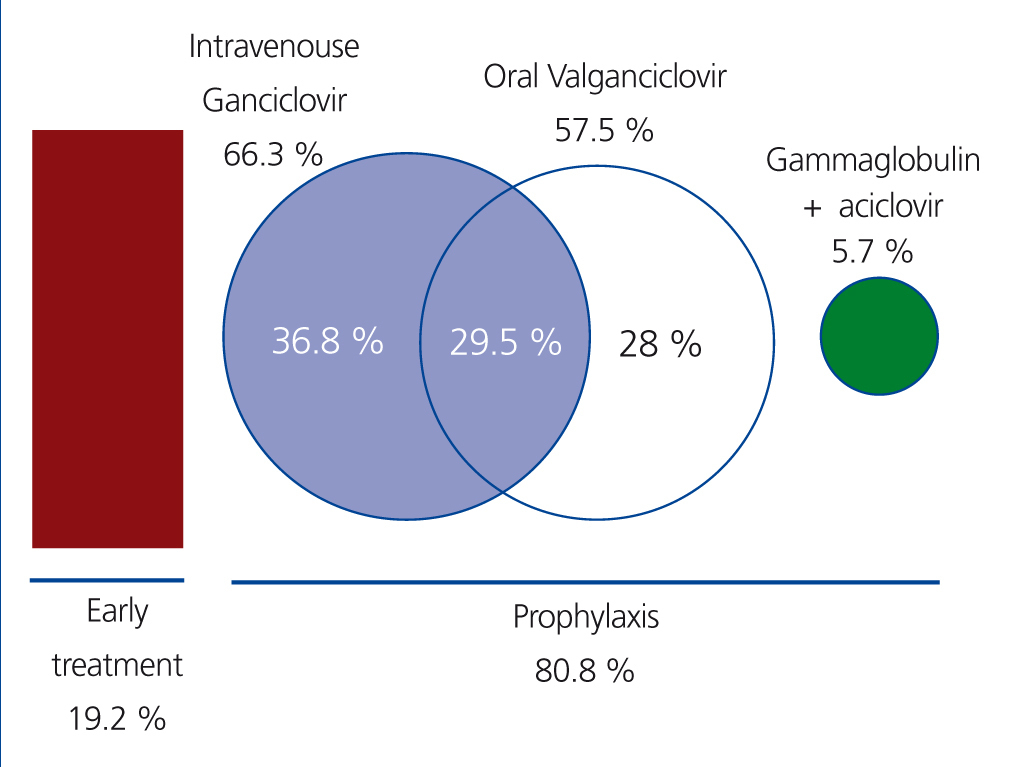
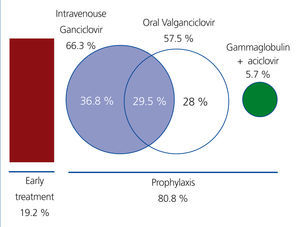
In 19.2% of recipients, only preemptive treatment was provided, with no anti-CMV prophylaxis. Prophylaxis was administered in the remaining 80.8% of cases (193 recipients) during a mean time of 65.5 days (range: 7-180 days; standard deviation: 50 days). Only 12 patients (5%) received prolonged prophylaxis for a 6-month period. All patients considered to be at high risk received chemical prophylaxis.
After prophylaxis was finalised, preemptive treatment was continued in these patients during the first 6 months post-transplantation.
The most commonly used medication was intravenous (i.v.) ganciclovir, which was administered in 66.3% of all recipients who received prophylaxis (n=128) and was the only form of treatment (during a mean time of 10 days) in 36.8% of these patients (n=71); this was followed sequentially by oral valganciclovir (57.5% of patients after treatment with i.v. ganciclovir) (Figure 2). In 5.7% of cases (n=11), prophylaxis was administered in the form of i.v. gammaglobulin for 10 days, followed by oral aciclovir for another 80 days.
Immunosuppressant treatment was administered in the form of induction therapy with antiCD-25 in 75.3% of cases (n=180), thymoglobulin in 23.8% (n=57), and monoclonal antibodies against mature lymphocyte CD-3 complex (OKT-3) was used in one patient. The majority of patients (98.3%) underwent quadruple therapy with an anti-calcineurin drug (96% tacrolimus [n=230] and 4% cyclosporine), mycophenolate mofetil (100% of patients), and prednisone (98.7%). We did not observe a significant correlation between any of these treatments and the incidence of CMV.
The diagnosis of CMV was made using antigenaemia tests in 34.3% of cases and PCR in 36%, with no record of the method used in the remaining 29.7%. The periodicity of these tests was a mean 15 days during the first 2 months following transplantation, every 3 weeks during the third and fourth month following transplantation, and every month thereafter.
Cytomegalovirus infection and disease
CMV tests were positive at some point during patient evolution in 58 cases (24.26%). Of these, 42 were only diagnosed with infection, and the remaining 16 (6.7%) were diagnosed with CMV disease. The mean time period between transplantation and positive CMV test results was 92.5 days, with a range of 20-310 days (standard deviation: 73.6 days), and always occurred after prophylaxis was finished.
- In 27 patients, the CMV infection was a primary infection, and 23 of these (85.2%) were high risk pairs (D+/R-), 2 were D-/R-, and the remaining two were D unknown/R-; this relationship was statistically significant (P<.001) (Table 2). All of these patients had received prophylaxis against CMV: 14 with i.v. ganciclovir for a mean 13 days (10-14 days), with positive CMV tests results appearing a mean 65 days after transplantation; 5 received prophylaxis with oral valganciclovir for a mean 130 days (range: 90-180 days), with positive results in CMV tests a mean 169 days after transplantation; 6 were treated with both: i.v. ganciclovir followed by oral valganciclovir until reaching 100 total days of treatment; the remaining two patients were treated with specific gammaglobulin for 10 days followed by oral aciclovir until reaching a total treatment time of 80 days.
- Of the 31 remaining cases that were considered to be reactivations of disease, 21 had received some type of prophylaxis: 12 with i.v. ganciclovir for 10 days, 8 with oral valganciclovir for a mean 72 days (range: 14-90 days), and one with specific gammaglobulin (10 doses) followed by aciclovir until reaching 2 months of treatment.
- Of the 16 patients who had CMV disease, 13 had received prophylaxis, 6 of these for a duration of time shorter than 20 days and with i.v. ganciclovir, 6 received oral valganciclovir for 100 days, and 1 received gammaglobulin for 10 days followed by aciclovir until reaching a total of 60 days of treatment. The remaining 3 patients received preemptive treatment. In 9 patients, the CMV infection was a primary infection, and the remaining 7 were considered reinfections. As regards symptoms, 7 patients developed viral syndrome, 2 had probable gastrointestinal disease, and the remaining 7 were not evaluated. Four of the patients with CMV disease had required increased immunosuppression therapy prior to disease onset: 3 due to acute graft rejection (2 received steroid boluses and 1 received steroid boluses and thymoglobulin) and 1 due to recurrent post-transplant vasculitis (steroid boluses and cyclophosphamide). Another 2 patients with CMV disease had been diagnosed with post-transplant de novo diabetes prior to disease onset. We observed no relationship between graft rejection and CMV disease (P=.15), or between diabetic state and disease (P=.085).
All the cases of CMV disease were treated with i.v. ganciclovir with good evolution.
- Six patients had recurring infections: 5 of these had received prophylaxis with i.v. ganciclovir for less than 20 days, and the sixth had received preemptive treatment. None of these patients had experienced episodes of rejection, diabetes, or intensified immunosuppression therapy.
- We did not observe a significant relationship between having received prophylactic treatment and the appearance of CMV (P=.656) or CMV disease (P=.95), but there was a correlation between the application of prophylactic treatment for less than 20 days post-transplantation (P<.05) and with the sole use of i.v. ganciclovir in prophylactic therapy (P<.05).
Acute rejection/diabetes
Thirty-two patients (13.4% of the total study sample) had at least one episode of acute rejection, and six of these suffered a second episode as well. Eleven of these (34.3%) also developed CMV infection or disease: in four of these, the CMV infection occurred prior to rejection (3 high risk D+/R- and 1 D+/R+), and in the other seven, CMV appeared after rejection. In these cases, the patients had received anti-rejection treatment: 3 steroid boluses in four patients, 7 doses of thymoglobulin in 1 patient, and both types of treatment in succession in 2 patients.
Nine patients were diagnosed with post-transplant de novo diabetes during the first year of follow-up; the diagnosis was made a mean 34.3 days after the intervention. Four of these cases also involved the appearance of positive test results for CMV, all of which occurred after the onset of diabetes.
We also failed to observe a significant relationship between acute rejection and CMV infection, the use of thymoglobulin, or diabetes.
We did not observe any cases of resistance, lymphoproliferative disease, or graft losses due to CMV infection.
DISCUSSION
We believe that our study provides a major contribution to this field of medicine due to the large number of paediatric patients evaluated as compared to other studies. In recent years, not even the data from the North American Paediatric Renal Trials and Collaborative Studies (NAPRTCS)9 have specified the incidence of CMV, and the article by this group regarding CMV prophylaxis, upon which many later studies have been based, dates back to 1997 and only involved hospitalised patients.10 In addition, our study truly reflects the situation of these patients in Spain due to our inclusion of 5 different paediatric nephrology units out of the 7 currently active in the field of paediatric renal transplants (these institutions carry out virtually all of such operations). Even so, we recognise that there is a certain loss of validity due to the fact that this was a retrospective study performed on a range of data derived from different centres that utilise different strategies and attitudes when dealing with CMV, the focus of our study.
In the few studies carried out among paediatric patients that have been published in the medical literature in recent years, the number of recipients with negative CMV serology test results at the moment of transplantation varies from approximately 55%11,12 to values as high as 87%.13 In our study, 54% of patients were CMV negative. This percentage among paediatric patients was much higher than in studies concerning adult patients (as expected), which range between 11%14 and 29%.15
As regards the prevalence of high-risk recipients (D+/R-), which constituted 26% of all patients in our study, this rate is slightly lower than the rates published in other paediatric studies, which range between 27.2%11 and 42%.13 However, the percentage of low-risk recipients (D-/R-) in our study was 24%, a mid-range value when compared to rates reported in previous studies (12%10 to 45%11).
The threshold value of antigenaemia or DNA-aemia for considering there to be a CMV infection and require preventative treatment was not evaluated, given the impossibility of super-imposing the results obtained from the different centres due to the wide range of methods used for diagnosis, as has already been mentioned.16,17 The periodicity of measurements, although determined according to the protocol at each unit, was quite similar across the different hospitals. It is not difficult to perform this type of monitoring of paediatric patients, who generally are evaluated frequently and at the same centre where the transplant was performed.
We also did not differentiate between reinfection and reactivation in the case of patients with positive CMV serological test results prior to transplantation and later detection of CMV, since the virus strain was not defined.
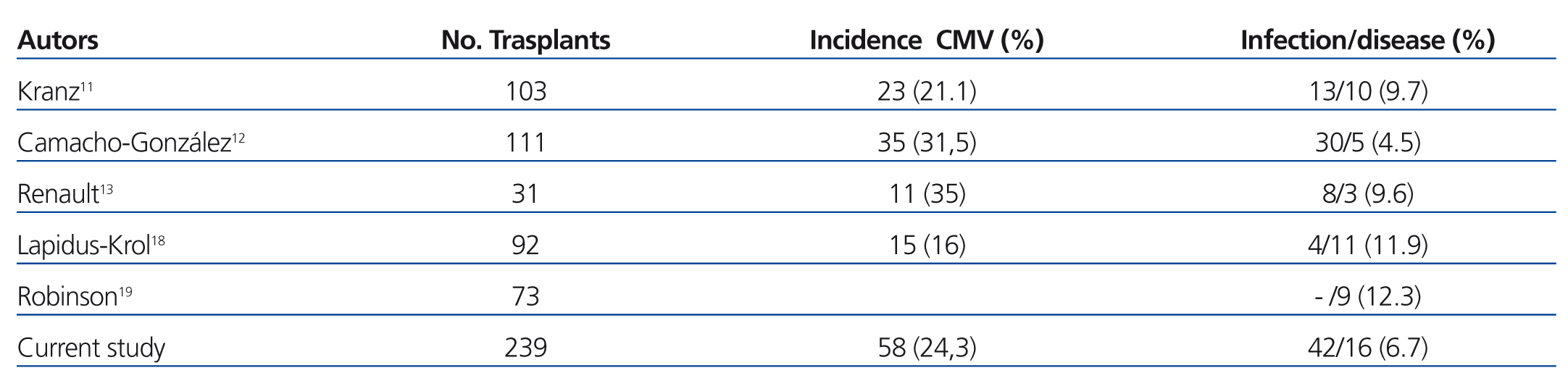
The incidence of CMV infection/disease in our patients during the first year following transplantation was 24.26% (58 cases out of the 239 transplants evaluated), which is lower than the incidence rate reported by other paediatric groups (Table 3).11-13,18,19 It is difficult to draw conclusions regarding which factors influence this incidence rate, given the variety of different strategies used to treat paediatric patients with CMV, not only in the different studies published, but even between patients in a single study. In this manner, the incidence rate decreases to 13% in small cohorts of paediatric patients that exclude low-risk individuals and provide prophylactic treatment to all individuals.18 In our study, we believe that the low incidence of CMV disease was due to the fact that all patients either received prophylactic treatment or preemptive treatment, with preemptive therapy even commencing after chemical prophylaxis was finalised.
It has been shown both in adults4,14,16 and the paediatric population11,12 –and we have again confirmed this in our study- that the incidence of CMV is greater in high-risk recipients, as well as the fact that positive donor serological test results are an independent risk factor for CMV infection.11,12 We have also shown that perhaps (this should be a focus of future studies) the use of prophylaxis would benefit recipients of organs from positive donors, regardless of the serology of the recipient. On the other hand, we did not observe a relationship between the use of prophylaxis and CMV infection, perhaps due to the large number of patients that received this type of treatment, except for cases where prophylaxis was kept for less than 20 days. This coincides with the significant relationship observed between prophylaxis with i.v. ganciclovir as a sole medication and only used during the first two weeks, with a 35.2% incidence rate of CMV within the group of patients who received prophylaxis (P.05). This incidence, which is greater than the general incidence of CMV in the overall study population, is close to the upper range of CMV rates (52.4%) reported in other studies in which patients received i.v. ganciclovir as the sole form of prophylaxis.20 This significance may not be related so much to the medication used, but is more probably related to the early use of prophylaxis and its short duration. In fact, studies have already reported evidence that supports delaying the start of prophylaxis until after the second week post-transplantation in order to allow for the specific immunity of the recipient to develop.21
To conclude, the current prevention strategy for CMV disease in paediatric recipients of renal transplants, whether through preemptive treatment or prophylaxis, maintains the incidence rate of this type of infection at similar values to those reported from other groups working with paediatric patients, without producing graft loss or severe disease during the first year following transplantation, although there is a greater incidence of infection when prophylaxis is administered for less than 3 weeks. We should evaluate whether extending the duration of prophylactic treatment to 6 months might improve these results, and the influence of this strategy on late indirect effects.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
KEY CONCEPTS
1. Approximately 54% of the paediatric population that receives a renal transplant in Spain does not have pre-transplant antibodies against CMV.
2. The mean incidence of CMV infection in cases of paediatric renal transplants in Spain is 24%, with an incidence of disease of approximately 7%.
3. CMV is associated with the situation of D+/R- (high-risk recipient) (P<.001) and with positive serology tests results in the kidney donor (P<.001) regardless of recipient status.
4. 25% of the population is at a high risk (D+/R-) for CMV infection after transplantation.
5. Currently, the primary preventative strategy is chemical prophylaxis, with a mean duration of 65 days.
6. Graft loss or patient death due to this aetiology in the first year following transplantation are rare.
Figure 1. Patients included in the study categorised by hospital
Figure 2. Preventive treatment strategy
Table 1. Studied variables
Table 2. Incidence of cytomegalovirus according to donor/recipient serological status
Table 3. Comparison with other studies