Introducción: En el año 2006, la Sociedad Española de Bioquímica Clínica y Patología Molecular (SEQC) y la Sociedad Española de Nefrología (S.E.N.) elaboraron un documento de consenso para facilitar el diagnóstico y seguimiento de la enfermedad renal crónica mediante la incorporación de la estimación del filtrado glomerular (FG) en los informes del laboratorio. La implementación y adhesión a las recomendaciones de dicho documento son desconocidas. Métodos: Encuesta dirigida a los laboratorios clínicos españoles realizada durante el período 2010-11, a través del correo electrónico o de contacto telefónico a los laboratorios participantes en el Programa de Garantía Externa de la Calidad de la SEQC, laboratorios de los hospitales incluidos en el Catálogo Nacional de Hospitales 2010, laboratorios de Atención Primaria y laboratorios privados. Resultados: 281 laboratorios respondieron a la encuesta. El 88,2% informaban la estimación del FG: el 61,9% mediante la ecuación MDRD y el 31,6% mediante la ecuación MDRD-IDMS. El 42,5% de los laboratorios aportaban el FG siempre que se solicitaba la medida de creatinina plasmática, y el resto sólo tras solicitud expresa de éste o asociado a perfiles analíticos. El 50,6% informaban cualquier valor de FG, mientras que el 46,2% sólo con el valor exacto los filtrados inferiores a 60 ml/min/1,73 m2. En el 56,3% de los casos, el valor del filtrado se acompañaba de un comentario interpretativo de éste. Conclusiones: Aunque un elevado porcentaje de laboratorios de nuestro país ha implementado el FG en sus informes, su uso no está generalizado, y aspectos como el tipo de ecuación utilizada y la correcta expresión de los resultados del FG no siempre están acordes a las recomendaciones existentes.
Introduction: In 2006 the Spanish Society of Clinical Biochemistry and Molecular Pathology (SEQC) and the Spanish Society of Nephrology (S.E.N.) developed a consensus document in order to facilitate the diagnosis and monitoring of chronic kidney disease with the incorporation of equations for estimating glomerular filtration rate (eGFR) into laboratory reports. The current national prevalence of eGFR reporting and the degree of adherence to these recommendations among clinical laboratories is unknown. Methods: We administered a national survey in 2010-11 to Spanish clinical laboratories. The survey was through e-mail or telephone to laboratories that participated in the SEQC’s Programme for External Quality Assurance, included in the National Hospitals Catalogue 2010, including both primary care and private laboratories. Results: A total of 281 laboratories answered to the survey. Of these, 88.2% reported on the eGFR, with 61.9% reporting on the MDRD equation and 31.6% using the MDRD-IDMS equation. A total of 42.5% of laboratories always reported serum creatinine values, and other variables only when specifically requested. Regarding the way results were presented, 46.2% of laboratories reported the exact numerical value only when the filtration rate was below 60mL/min/1.73m2, while 50.6% reported all values regardless. In 56.3% of the cases reporting eGFR, an interpretive commentary of it was enclosed. Conclusions: Although a high percentage of Spanish laboratories have added eGFR in their reports, this metric is not universally used. Moreover, some aspects, such as the equation used and the correct expression of eGFR results, should be improved.
INTRODUCTION
In 2006, the Spanish Society of Nephrology (S.E.N.) commenced a strategic action programme for Chronic Kidney Disease (CKD)1 with the goal of establishing a series of protocols for the nationwide treatment and management of CKD that would: a) define the epidemiological reality of CKD in Spain; b) facilitate the detection of patients with CKD or at risk of developing it; c) optimise the treatment of CKD in all stages of its progression; and d) avoid late diagnosis and treatment of CKD patients in nephrology departments. Among its different implications, this programme involves the incorporation of glomerular filtration rate (GFR) estimates in all Spanish laboratory results. With this purpose, the S.E.N. and the Spanish Society of Clinical Biochemistry and Molecular Pathology (SEQC) elaborated a consensus document of recommendations for using equations for estimating glomerular filtration rates in adults,2 the primary objective of which was to facilitate the detection of CKD by promoting the use of estimated GFR through calculation based on established equations in all clinical laboratory reports whenever plasma creatinine levels were requested. In addition, this document attempted to homogenise other aspects pertaining to the use of equations, such as the type of equation to use, the format used to express results, and interpretation.
In order to evaluate the level of adherence to the recommendations in this document, the Renal Function Commission (CFR) of the SEQC carried out a survey in 2010-2011 amongst the clinical laboratories in Spain.
MATERIAL AND METHOD
During 2010 and the first semester of 2011, we administered a survey to Spanish clinical laboratories in order to evaluate the level of implementation of equations for estimating GFR in clinical laboratory reports, as well as other aspects related to the use of this variable. The questionnaire (Appendix) included a total of 13 questions covering 5 different aspects: a) the location and type of requests received by each laboratory; b) the methodology used for measuring creatinine; c) the use or lack thereof (including reasons) of equations for estimating GFR; d) how the estimated GFR results are expressed; and e) the laboratory’s interest in participating in a specific quality control programme for GFR. The questions regarding methodological aspects of creatinine measurements were incorporated into the survey at the end of 2010 (questions 2.1 and 2.2, Appendix) based on initial responses that indicated the need for assessing the concordance between the method used for measuring creatinine and the equation selected for estimating GFR. The question of whether each laboratory would be interested in participating in a specific quality control programme had the objective of evaluating the viability of a similar programme to that currently in place in the United Kingdom, in which several vials of human serum with specific standardised creatinine concentrations are periodically sent to each clinical laboratory for analysis.
In the absence of a comprehensive directory of clinical laboratories, we opted for the use of information on the participating laboratories in the XXXI external biochemical quality control programme of the SEQC in 2010.3 This programme included all public, private, emergency, and partnership clinical laboratories in the different autonomous communities of Spain.
During the first semester of 2010, the survey was sent to all participating laboratories in this programme (n=666), but given the low response rate (17%), we decided to contact each laboratory by telephone through the information included in the National Hospitals Catalogue, which was updated on 31 December 2010 (CNH 2010).4 Although this catalogue only included hospitals, we also took into account primary care and private laboratories through personal contacts with members of the CFR. Given the large number of centres and the difficulty to obtain information, towards the end of 2010 we decided to administer the survey to hospitals with more than 100 beds (n=470), and to exclude psychiatric, geriatric, and extended stay hospitals, since these tend not to have their own laboratories, which resulted in a theoretical population of 361 hospital laboratories. The data collection process from the surveys was concluded in mid-2011.
We performed all statistical analyses of the data using SPSS statistical software for Windows, version 12.0 (SPSS Inc., 1989-2003).
RESULTS
Laboratory characteristics and representativeness
We obtained a total of 281 responses, although one hospital was excluded since it did not adequately provide GFR as estimated using equations. For this reason, the statistical analysis was based on a total sample size of 280 responses. The characteristics of each laboratory and the origin of the requests they received are summarised in Table 1.
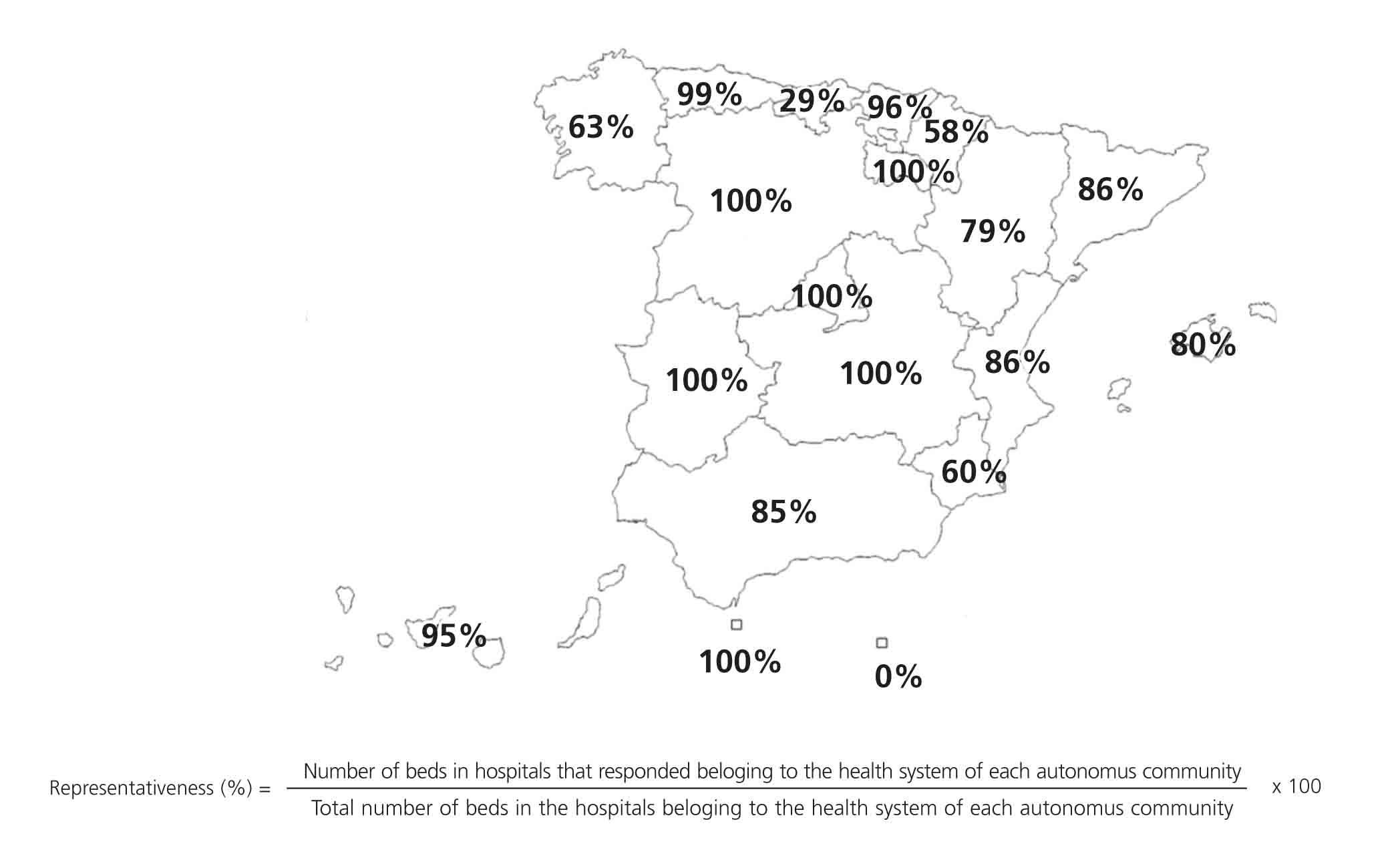
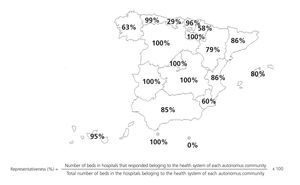
In order to evaluate the representativeness of our sample, we only took into account the hospitals within the health system of each autonomous community, calculating the number of beds at each centre that responded to the survey as compared to the total number of beds. The results are shown in the Figure, separated according to autonomous community.
Method for creatinine measurements
Since the two questions regarding this topic were incorporated into the second version of the survey, we only have responses from 125 laboratories. The methods used were: calibrated Jaffe kinetic method (62 laboratories), conventional Jaffe kinetic method (48 laboratories), dry slide enzymatic method (10 laboratories), and the enzymatic method (5 laboratories).
The use of equations for estimating glomerular filtration rate and the format for expressing results
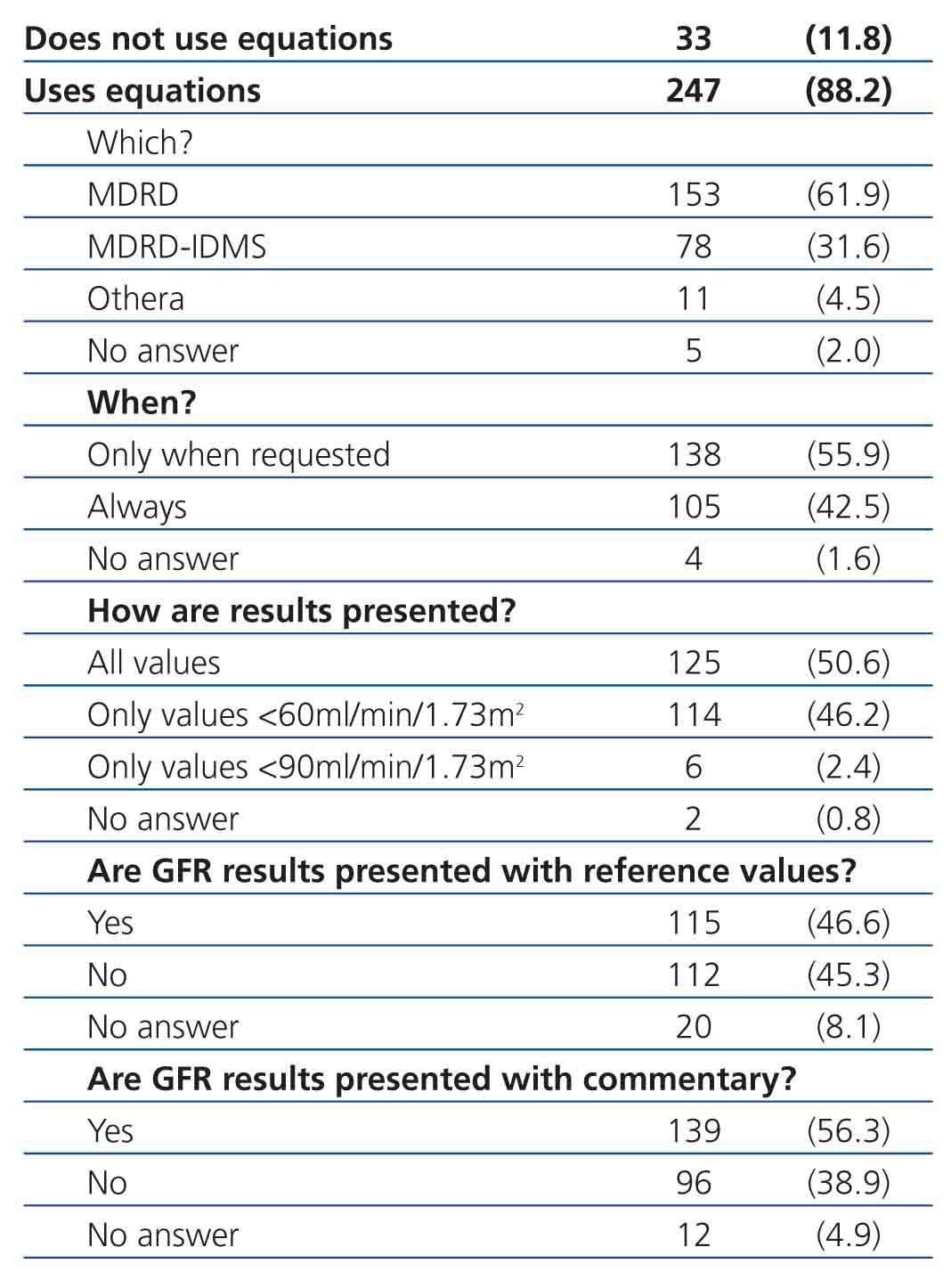
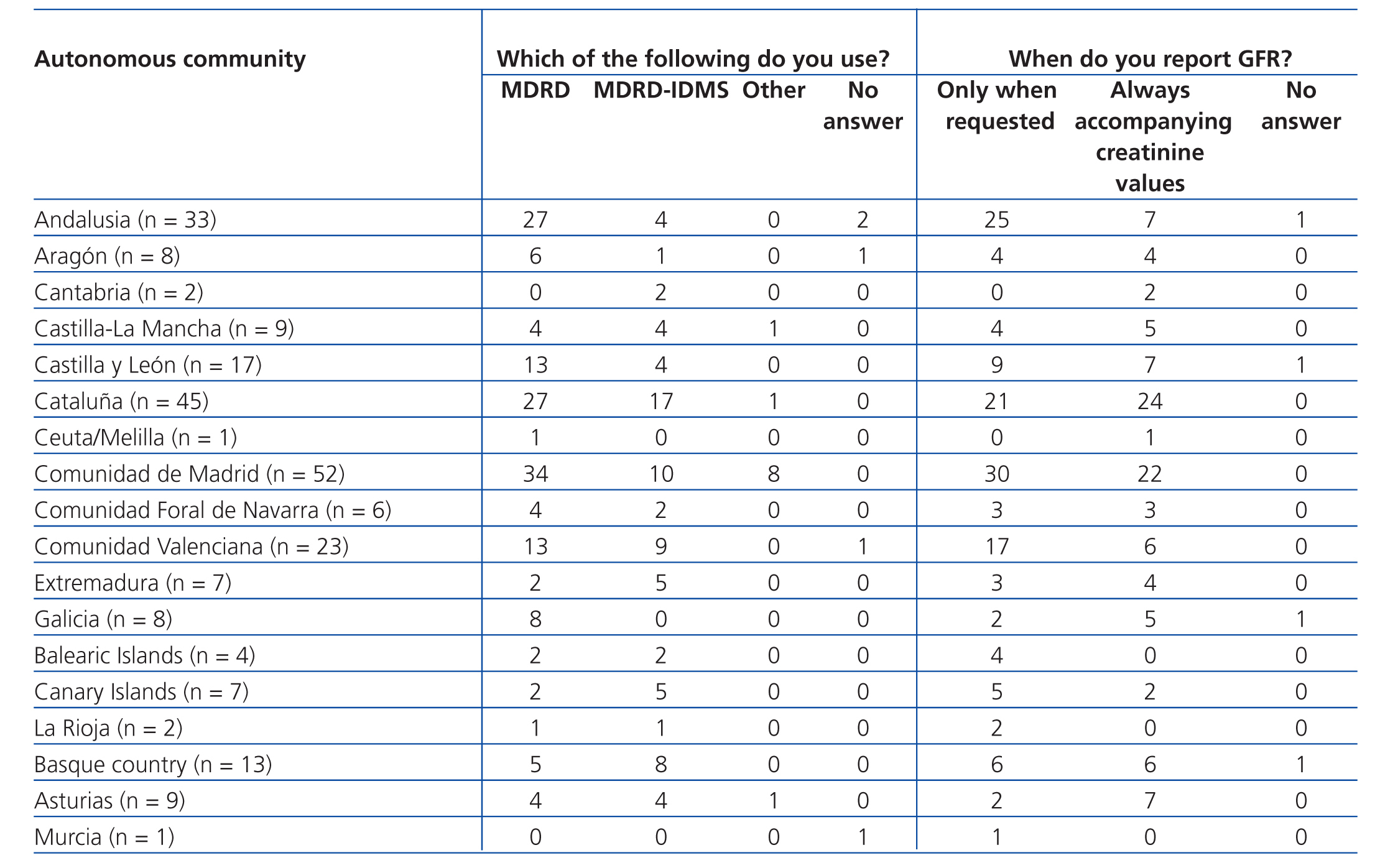
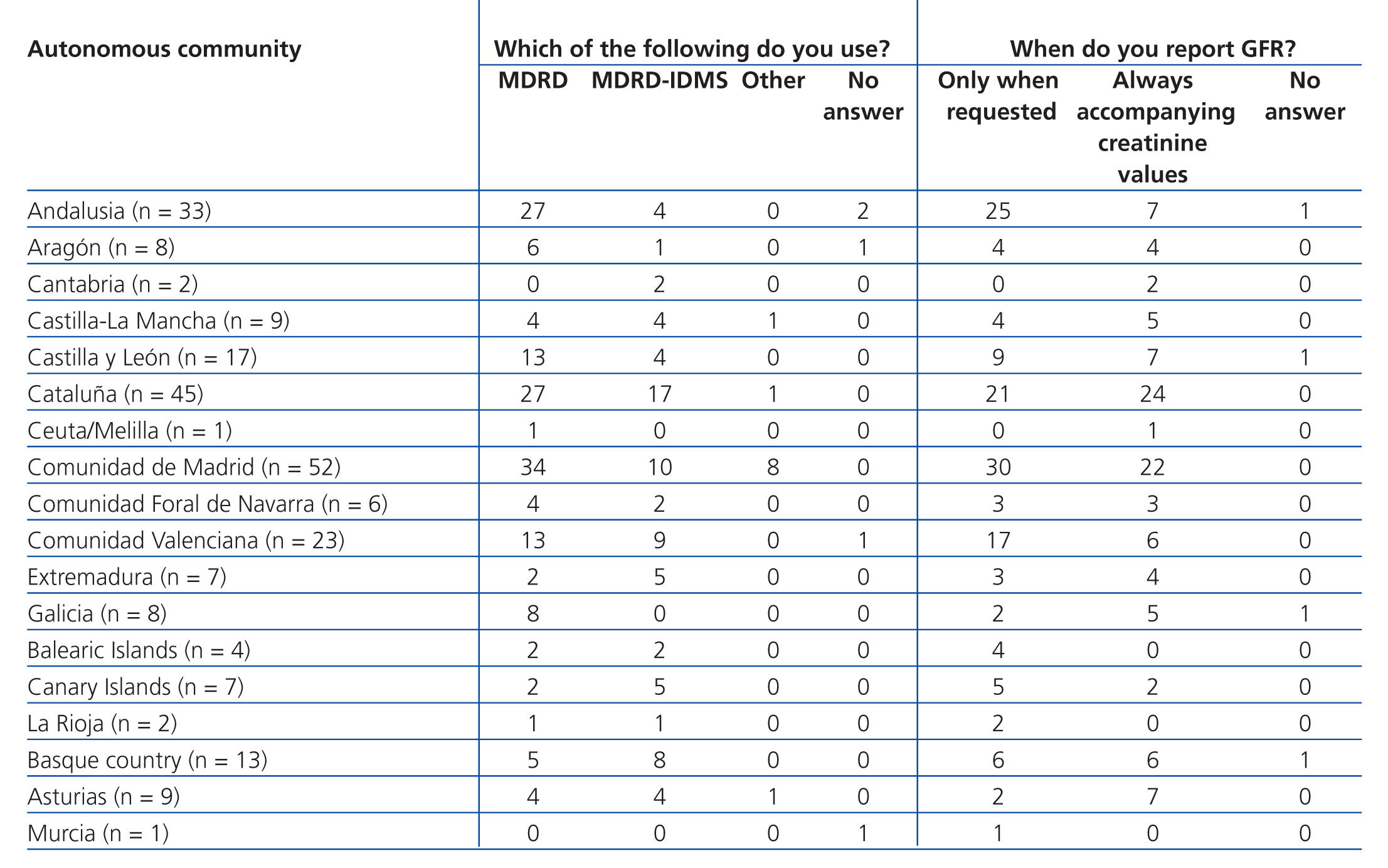
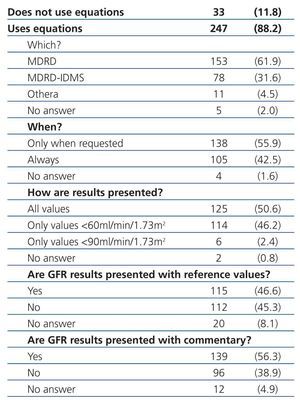
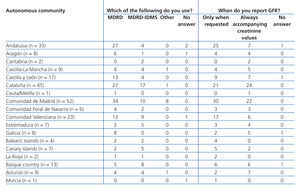
Equations for estimating GFR were used by 88.2% of participating laboratories. The MDRD equation from non-standardised creatinine measurements was the most commonly used method for calculating GFR (61.9%), followed by the MDRD-IDMS for standardised creatinine measurements (31.6%) and other equations (4.5%), including the Cockcroft-Gault, CKD-EPI, Schwartz, and Counahan-Barratt methods. GFR was not reported by 11.8% of laboratories; the most common reasons for this were (taking into account that more than one response could be selected): the clinical departments had not requested these values (n=17), the laboratory database system did not allow for incorporating these values into the report (n=5), the equations were not considered to be sufficiently valid (n=2), their use was not believed to be needed (n=1), other non-specified reasons (n=4), and no response to the question (n=8). Table 2 displays overall results in terms of whether or not laboratories used equations for estimating GFR, the type of equation used, and the manner in which results were expressed, and Table 3, Table 4A, and Table 4B show the results separated by autonomous community.
In laboratories for which we were given information regarding the methods, equipment, and in vitro diagnostic tools used for measuring creatinine values (n=125), we reviewed the appropriateness of the type of equation used. A total of 46.8% did not use the appropriate equation, 28.4% did use the appropriate equation, and 24.7% could not be assessed due to a lack of necessary information.
Participation in an external quality control programme for glomerular filtration rate
As regards the interest of each laboratory in participating in a specific external quality control programme for measuring GFR, 69% of laboratories were interested, 15% were not interested, and 16% did not respond to the question.
DISCUSSION
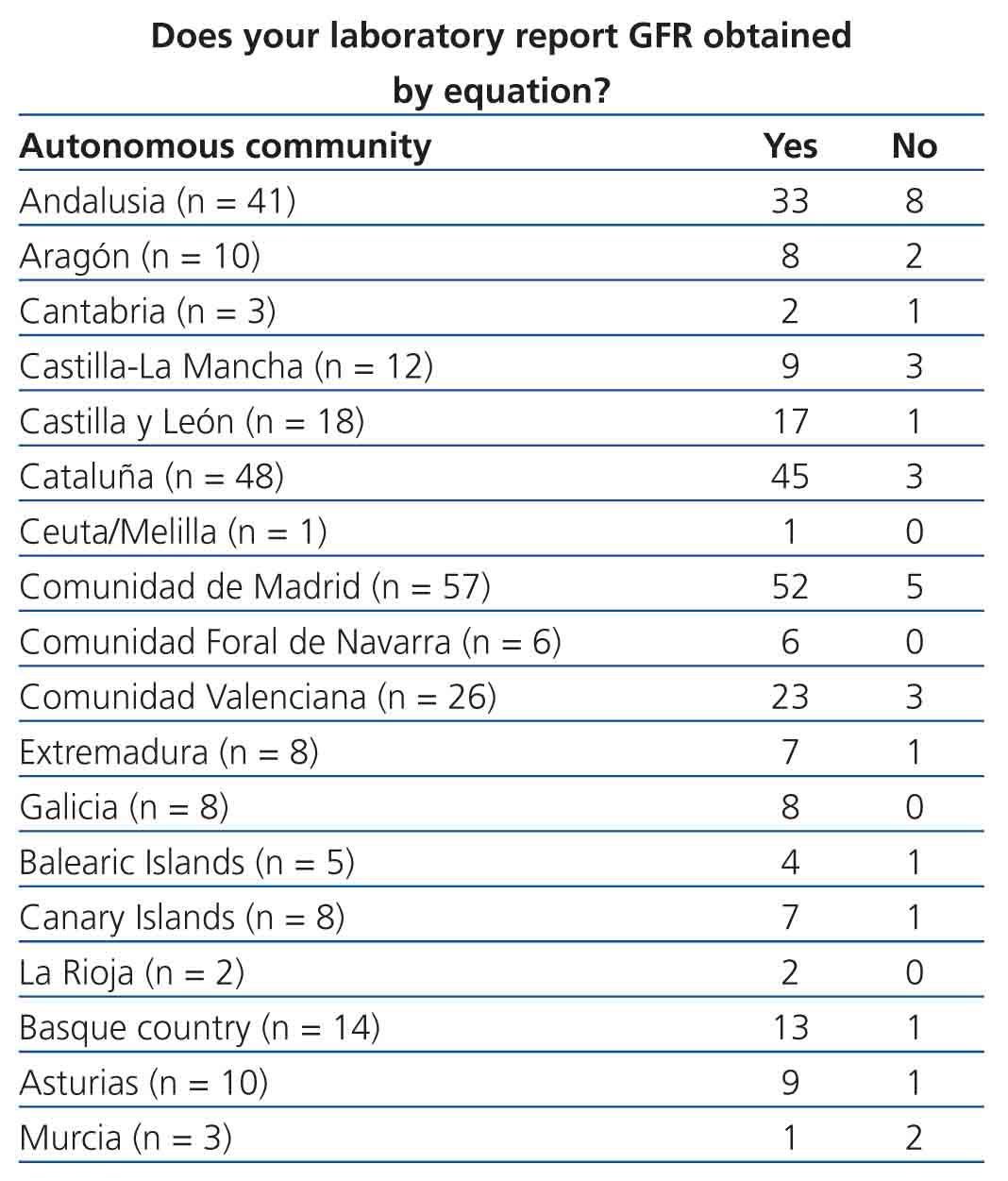
One of the main problems of this survey was to objectively determine how representative our sample was of the overall situation in Spanish laboratories. The lack of a specific global directory of clinical laboratories led us to use the CNH 2010 as our source of information, in which non-hospital laboratories are not represented. In order to reduce the impact of this issue, we widened our search to personal contacts of members of the CFR, but the exact scope of the reach of this method is not quantifiable. We determined how representative our sample was by comparing the number of beds at each centre in order to assess the total health care provided by each hospital and, consequently, the population attended by each respective laboratory, but this method has certain limitations. For example, in Cataluña, primary care laboratories are not associated with hospitals and so have not been included in the calculation of representativeness. However, the results obtained can be considered to be a good approximation to the current situation in our country, since we have collected the information from, on average, more than 80% of hospital beds within the health systems of each autonomous community from centres included in the CNH 2010.
The use of equations for estimating GFR in Spanish clinical laboratories has increased in the last 5 years. The results of surveys administered by the CFR in 2006 and 2008,5,6 while not completely representative and probably somewhat biased due to poor participation (71 and 93 laboratories, respectively), indicated that 30% and 88% of laboratories were using equations for estimating GFR, respectively.
Similar surveys administered by the National Kidney Disease Education Program (NKDEP) during 2006-7,7 the College of American Pathologists of the United States (CAP) in 2010,8 and in the United Kingdom in 2010,9 indicated that 38%, 80%, and 83% of laboratories, respectively, provide estimated GFR through the use of equations.
The most commonly used equations are the MDRD (61.9%) and MDRD-IDMS (31.6%). During the survey period, the process of standardising creatinine values was substantially advanced, and so the higher use of MDRD (non-standardised methods) versus MDRD-IDMS (standardised methods) suggests that, in some cases, the appropriate equation was not used. The reason for this could be that the introduction of standardised creatinine procedures in Spain has been progressive, and it is possible that clinical laboratories that introduced the calculation of GFR through the MDRD equation at one point have not made the switch to the MDRD-IDMS, even after implementing standardised methods. Similar situations have been detected, although not certified, in surveys administered by the CAP. It is important that laboratories use the MDRD-IDMS equation when standardised methods are used, since creatinine values decrease by 10%-20% in these cases, which can lead to an over-estimation of GFR. With the objective of facilitating a convenient use of equations for estimating GFR, the CFR has included a list of the most commonly used methods for measuring creatinine in our country on their website, indicating the appropriate equations to use in each case.10 Other equations for the adult population, such as the 6-variable MDRD (MDRD-6) and the Cockcroft-Gault equation, are not commonly used (1 and 6 laboratories, respectively), as is the case for the Schwartz equation (2 laboratories) and the Counahan-Barratt equation (1 laboratory). The use of the MDRD-6 implies a substantial increase in costs (by including serum urea and serum albumin measurements) without significantly improving the capacity for detecting CKD. The use of weight in the Cockcroft-Gault equation also implies added difficulties. In addition, both the MDRD-6 and the Cockcroft-Gault equations have not been modified for methods incorporating IDMS, and so should no longer be used.
The majority of laboratories use procedures for measuring creatinine based on the kinetic Jaffe method, and only a few use enzymatic methods. The determining factor is the lower cost of the Jaffe method, despite the problems with specificity that arise in a substantial proportion of the population, specifically in groups such as the elderly and children. This must be taken into account when deciding what equation to use, since the Schwartz equation standardised for paediatric patients has been reformulated,11 and is restricted to the use of standardised enzymatic methods.
One aspect we wish to highlight is that the use of equations for estimating GFR has not been standardised, and the majority of laboratories surveyed (55.9%) only provide this value when explicitly requested to do so, often in the context of concrete laboratory profiles or based on the requesting department (nephrology or internal medicine), which does not comply with the current national and international guidelines.
As regards the format in which laboratories express test results, more than 50% provide the exact number obtained. The recommendations indicate that GFR values greater than 60ml/min/1.73m2 should be expressed as >60ml/min/1.73m2, and not as the exact value obtained. This is due to the lack of precision and accuracy of equations in estimating GFR at values greater than 60ml/min/1.73m2, and the fact that, above these values, estimated GFR is not diagnostic of CKD. Recently, the Chronic Kidney Disease Epidemiology Collaboration developed a new equation (CKD-EPI) that provides the advantage of a greater level of precision in calculating GFR values >60ml/min/1.73m2, which has led its creators to recommend providing the exact value when obtained using this equation. Although no scientific societies currently recommend substituting the MDRD-IDMS with the CKD-EPI equation, laboratories that wish to provide precise values at level greater than 60ml/min/1.73m2 should consider switching to the CKD-EPI equation. In this context, the NKDEP warns that the lack of precision in the available measurement methods when creatinine concentrations are low (corresponding to elevated GFR values) can be a limitation in determining exact GFR values.12 In all probability, it would be more useful to promote the use of GFR and albumin/creatinine ratios in urine samples for the detection of CKD, rather than debating whether to use the MDRD-IDMS or CKD-EPI equations.
Approximately 50% of laboratories include a reference interval for GFR, and approximately 40% do not provide commentary that aids in the interpretation of the GFR estimate. In this sense, we believe that clinical laboratories could provide valuable assistance in interpreting results, above all for health professionals that are not specialised in nephrology.
The reasons indicated by laboratories not to provide GFR values are primarily the absence of a specific request for these values by clinical departments and difficulties in the laboratory’s digital information databases. We believe that justifying the absence of these values with the lack of a specific request from attending physicians is unacceptable in current medical practice. The issues with laboratory databases are probably rectifiable in the majority of cases.
Participation in a specific external quality control programme for GFR would facilitate a direct assessment of accuracy and precision for each different method of measurement, and the calculation of which specific factors must be adjusted for each method, with the objective of reducing the variability of GFR values obtained by each different laboratory.13 A significant percentage of laboratories do not consider that the estimation of GFR should warrant such a quality control programme. The reasons could be a lack of information regarding the specific characteristics and objectives of the programme, economic considerations, or the concept of GFR as just another baseline formula or indicator of patient health rather than a parameter that should be evaluated in a quality control programme. Currently, the Quality Commission of the SEQC is developing a pilot programme for using human serum samples that have been evaluated using an IDMS reference procedure.
In conclusion, although a large proportion of clinics in our country have implemented the use of equations for estimating GFR to evaluate renal function, the use of these equations has not been standardised throughout the national health network; in more than 50% of cases, it is only provided when explicitly requested, and does not always accompany creatinine concentration values. Issues such as which equation to use and the correct expression of GFR results are important questions that must be updated in order to comply with current recommendations.
Acknowledgements
The Renal Function Commission of the SEQC would like to thank all laboratories that answered the survey for their collaboration.
Conflicts of interest
The authors have no potential conflicts of interest to declare.
Table 2. Use of equations for estimating glomerular filtration rate and how results are expressed in Spanish laboratories
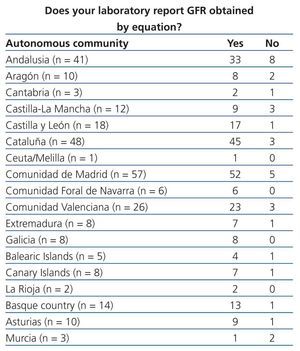
Table 3. Implementation of equations for estimating glomerular filtration rate in laboratory reports. Results categorised by autonomous community
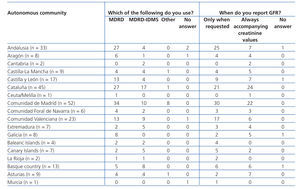
Table 4 A. Use of equations for estimating glomerular filtration rate. Results categorised by autonomous community
Table 4 B. Use of equations for estimating glomerular filtration rate. Results categorised by autonomous community
Figure 1. Representativeness of the survey, calculated from the number of beds at each hospital within the health system of each autonomous community
Survey model
11375_19157_32391_en_w47771263115ref.1137514906_11375_19115_28361_es_11375_tabla1_en.doc
Table 1. Characteristics of the participating laboratories