Introduction: Important differences in patient survival exist between peritoneal dialysis (PD) and haemodialysis (HD). Several different studies have shown that PD yields a better survival rate than HD in the first and second years of treatment, especially in younger patients and non-diabetic patients with low comorbidity, whereas HD produces better results in diabetic patients, elderly patients, and in patients with greater comorbidity. In recent years, interesting changes have occurred in PD units in the Canary Islands, such as the introduction of peritoneal dialysis solutions with bicarbonate dialysate and low content of glucose degradation products, extended use of automated dialysis, and continuity of physicians and nurses in PD units, in addition to enhancing visits for advanced chronic kidney disease (ACKD). Objective: This situation led us to perform our study with the primary objective of comparing medium-term survival among incident dialysis patients on HD versus PD in recent years in the Canary Islands, and as a secondary objective, to compare survival between these two types of dialysis by subgroups as defined by age, sex and diabetes. Material and methods: This was a retrospective cohort study comparing survival between HD and PD patients starting dialysis in the Canary Islands between 01/01/2006 and 31/12/2009, with adjustment based on the propensity score analysis. We analysed data from the RERCAN database, which collects data on demographic variables, changes in type of dialysis, province and hospital of the patient, and mortality and its causes. We calculated Kaplan-Meier estimates of survival based on the overall population and stratified by age, sex and diabetes. We applied a Cox proportional hazards model for survival to estimate the relative mortality risk of PD compared with HD, using as independent variables: age, sex, quartiles of propensity score, the province of the patient, and diabetes. Finally, we applied a Cox model with time-dependent effects, using as a fixed risk factor the initial type of dialysis in order to assess the effect of PD versus HD on short and medium-term survival. Results: The cohort included 1469 patients (173 PD and 1296 HD), with a mean age of 62.5 years, 65% male. Mean follow-up was 16.2±12.4 months. Factors associated with greater probability of choosing PD were younger age and living in the province of Las Palmas. The cumulative mortality in the intention to treat (ITT) analysis was 27.1% in the HD group and 8.7% in the PD group, P<.0001. The cumulative probability of survival by ITT using PD vs HD was 96.6% versus 89% at 6 months (P<.001), 96% versus 80% at 12 months (P<.001), 90% versus 65% at 24 months (P<.001), 82% versus 58% at 36 months (P<.001) and 73% versus 45% at 46 months (P<.001). In the subgroup analysis, survival was also higher in PD patients compared to HD patients both over and under 65 years old, in both diabetic and non-diabetic patients, and in both genders. The same analysis from the 90th day onward produced similar results. In the ITT analysis, the Cox-adjusted mortality risk for PD was 61% lower than for HD (RR: 0.398, 95% CI 0.237-0.669, P=.001), adjusted for age, diabetes, sex, patient’s province and propensity score. Broken down by years of survival on the technique used, the relative risk of death for PD compared with HD in the first year was also significantly lower (RR 0.509, 95% CI: 0.259-0.999, P=.049). From year 2 onwards, only age was a risk factor for mortality (RR: 2.785, 95% CI: 1.525-5.086, P=.001) and no differences were shown between the two dialysis techniques. Conclusion: In the Canary Islands, PD has demonstrated survival advantages over HD in the short and medium term. It is remarkable that this benefit was found in young and old patients, men and women, and diabetic and non-diabetic patients, and that this advantage was maintained even after years of being on dialysis.
Introducción: Existen importantes diferencias en los resultados de supervivencia de paciente y técnica entre diálisis peritoneal (DP) y hemodiálisis (HD) en las distintas series publicadas. Varios estudios han demostrado que la DP tiene mejor supervivencia que la HD en el primer y segundo año de tratamiento, sobre todo en los pacientes más jóvenes, no diabéticos y con menor comorbilidad, mientras que la HD parece mejor en los pacientes diabéticos, de más edad y mayor comorbilidad. En la Comunidad Canaria, en los últimos años se han ido introduciendo cambios asistenciales interesantes en las unidades de DP, como son la introducción de las soluciones de DP con baño de diálisis con bicarbonato y con bajo contenido en productos de degradación de la glucosa, la extensión del uso de la diálisis automatizada y la continuidad del médico y de la enfermera en las unidades de DP, además de la potenciación de las consultas de enfermedad renal crónica avanzada (ERCA). Objetivo: Esta situación nos condujo a realizar nuestro estudio con el objetivo principal de comparar la supervivencia a medio plazo entre pacientes incidentes en HD frente a DP en los últimos años en Canarias y como objetivos secundarios comparar la supervivencia entre dichas modalidades por subgrupos definidos por edad, sexo y diabetes. Material y métodos: Se trata de un trabajo de cohorte retrospectivo que compara la supervivencia entre HD y DP de pacientes que inician diálisis en la Comunidad Canaria entre el 1-1-2006 y 31-12-2009, con ajuste basado en el análisis de propensión. Se analizaron los datos de la base de datos RERCAN (Registro de Enfermos Renales de Canarias) que recoge variables demográficas, cambios de modalidad de diálisis, provincia y hospital de procedencia del paciente, mortalidad y causas de mortalidad. Se calcularon las estimaciones de Kaplan-Meier de supervivencia comparada en la cohorte global y por estratos definidos por la edad, sexo y diabetes. Aplicamos el modelo de riesgos proporcionales de Cox de supervivencia para estimar los riesgos relativos de mortalidad de la DP en comparación con la HD, utilizando como variables independientes de ajuste la edad, el sexo, el score de propensión por cuartiles, la provincia de procedencia del paciente y la diabetes. Finalmente, se aplicó un modelo de Cox estratificado en el tiempo (Cox time-dependent effects) usando como factor de riesgo fijo la modalidad inicial de diálisis, para valorar el efecto en la supervivencia, a corto y medio plazo, de la DP comparada con la HD. Resultados: La cohorte incluyó a 1.469 pacientes (173 en DP y 1.296 en HD), con una edad media de 62,5 años, el 65% hombres. El seguimiento medio fue de 16,2 ± 12,4 meses. Los factores asociados con una mayor probabilidad de elegir DP fueron la menor edad y la provincia de Las Palmas. La mortalidad acumulada, en el análisis por intención de tratar, fue en el grupo de HD del 27,1% y en el grupo de DP de 8,7 % (p <0,0001). La probabilidad acumulada de supervivencia por intención de tratar para DP y HD fue del 96,6 frente al 89% a los 6 meses (p <0,001), del 96 frente al 80% a los 12 meses (p <0,001), del 90 frente al 65% a los 24 meses (p <0,001), del 82 frente al 58% a los 36 meses (p <0,001) y del 73 frente al 45% a los 46 meses (p <0,001). En el análisis por subgrupos, la supervivencia fue también mayor en los pacientes en DP con respecto a los de HD tanto en los mayores de 65 años como en los menores, en los diabéticos y en los no diabéticos, y tanto en hombres como en mujeres. El mismo análisis a partir de los 90 días mostró resultados muy similares. En el análisis por intención de tratar, el riesgo de mortalidad ajustado por el modelo de Cox para la DP en comparación con la HD fue un 61% menor que para HD (RR: 0,398; IC 95%: 0,237-0,669; p = 0,001), ajustado para edad, diabetes, sexo, provincia y score de propensión. Desglosado por años de supervivencia en técnica, el riesgo relativo de mortalidad para la DP en comparación con la HD en el primer año fue también significativamente inferior (RR: 0,509; IC 95%: 0,259-0,999; p = 0,049). A partir del segundo año, sólo la edad se mostró como factor de riesgo de mortalidad (RR: 2,785; IC 95%: 1,525-5,086; p = 0,001) y no hubo diferencias entre las dos técnicas de diálisis. Conclusión: En Canarias, la DP ha demostrado ventajas de supervivencia a corto y medio plazo respecto a la HD. Resulta notable que este beneficio se ha constatado en pacientes jóvenes y de edad avanzada, diabéticos y no diabéticos, hombres y mujeres, así como que esta ventaja se mantenga incluso tras años después de aplicar la técnica.
INTRODUCTION
In spite of the technological innovations in renal replacement therapy for chronic kidney disease (CKD), important differences still exist in terms of survival results between peritoneal dialysis (PD) and haemodialysis (HD). Possible justification for this controversy may be found in initial patient comorbidity, the experience at the dialysis centre, and confounding factors for the clinical indications of each technique within the context of retrospective observational studies.1-7 Consequently, methodological differences, such as the absence of prospective study designs, small sample sizes, and the absence of statistical methods based on propensity scores in the majority of published studies could bias survival results.1,8-10 Propensity scores can be a very useful tool in this type of observational study, in which the distribution of clinical variables such as age, sex, and comorbidity can be very different between study groups, and are defined as the probability that a patient will be assigned to a given treatment group, in this case, type of dialysis, based on the clinical characteristics of the patient at that time.
In any case, several studies have shown that treatment with PD leads to better survival rates than with HD in the first and second years of treatment, above all in younger, non-diabetic patients with lower comorbidity, whereas HD appears to produce better results in diabetic, older patients with greater comorbidity.11-22
However, in recent years, interesting changes in health care have been introduced into PD units, such as PD solutions with bicarbonate dialysate and low content of glucose degradation products, extended use of automated dialysis and continuity of physicians and nurses in PD units, in addition to enhancing consultations for advanced chronic kidney disease (ACKD).
This changing situation has led us to carry out this study with the primary objective of comparing medium-term survival of incident dialysis patients on HD versus PD in recent years in the Canary Islands. As a secondary objective we will compare survival between these two different types of dialysis by patient subgroups as defined by age, sex, and diabetes.
MATERIAL AND METHOD
This is a retrospective cohort study comparing survival between adult HD and PD patients starting dialysis in the Canary Islands between 1 January 2006 and 31 December 2009. It was adjusted based on the propensity score analysis in order to mitigate the influence of the differences in baseline characteristics of the patients that chose one type of dialysis or the other.
We analysed data from the RERCAN database (kidney patient registry of the Canary Islands), which collects demographic variables such as age, sex, underlying disease, changes in type of dialysis, province and hospital of the patient, and mortality and its causes. Patients that were already on haemodialysis or had received transplants were excluded from the study.
Statistical Method
We compared the different parameters collected during the study between HD and PD patients. Continuous variables were first compared using Student’s t-tests, and differences between proportions were estimated using chi-square tests.
In order to account for the differences in baseline characteristics between patients, and thus reduce any possible bias in favour of one type of dialysis, we performed a propensity analysis, which was subsequently used as an adjustment variable in the Cox analysis. We estimated the propensity score for choosing PD at the start of dialysis using a logistical regression model that included all covariables that resulted predictive in the comparison of standardised differences in the baseline characteristics of patients based on the type of dialysis (Table 1 and Table 2).
The causes for halting follow-up were: patient death, kidney transplantation, loss of follow-up, patient transfer to another type of treatment, or the final date of the study, which was 31 December 2009.
The primary analysis focused on patient survival compared by dialysis type, according to intention to treat (ITT) analysis (starting at day 0). Secondary analyses included survival compared by dialysis type starting at the 90th day of treatment, and a stratified analysis based on age (using the median age of the sample, 65 years, as the cut-off point), sex, and diabetes.
We calculated Kaplan-Meier survival estimates based on the global sample and on the previously defined categories.
We then developed a Cox proportional regression model for survival in order to estimate the relative mortality risks of PD compared with HD, using as independent variables for adjustment: age, sex, quartiles of propensity score, the province of the patient, diabetes, and underlying disease. Finally, we applied a Cox model with time-dependent effects, using the initial method of dialysis as a fixed risk factor in order to assess the short and medium-term effects of dialysis (PD vs HD) on patient survival.18
We considered values to be statistically significant when P<.05. We used SPSS version 13.0 software for all statistical analyses (SPSS Inc., Chicago, USA).
RESULTS
The patient cohort included 1469 adults and 1235 of them (84%) survived more than 90 days after the start of dialysis. The mean age of patients was 62.5±15.3 years (range: 7-94, median: 65), and 65% were men. 11.8% of patients received PD at the start of dialysis (n=173 out of 1469). The mean follow-up period was 16.2±12.4 months (range: 1-47, median: 13, 75th percentile: 24 months). The underlying conditions were: chronic glomerulonephritis (7%), interstitial nephropathy (4.3%), diabetic nephropathy (44.3%), familial nephropathy (0.7%), ischaemic nephropathy (11.1%), unknown origin (15.3%), polycystic kidney disease (7.5%), systemic disease (2.6%), and other. The distribution of underlying conditions was similar in both treatment groups.
Table 1 shows the comparison of patient characteristics based on the type of dialysis started on day 0. PD patients were significantly younger, and were primarily from the province of Las Palmas. These factors associated with a greater probability of choosing PD (Table 2) were used to develop the propensity score using the beta values from the significant variables produced by the logistical regression. We distributed the propensity score values into quartiles: 26% of patients (n=380) had a score of +0.55 (score 4), 22% (n=323) had a score of 0 (score 3), 23% (n=336) had a score of -0.39 (score 2), and 29% (n=430) had a score of -0.94 (score 1). Higher scores (score 4 being the highest) indicated a greater probability that the patient would choose PD.
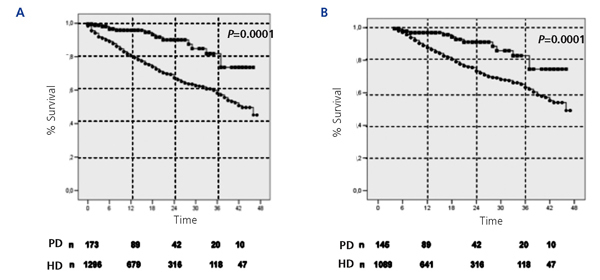
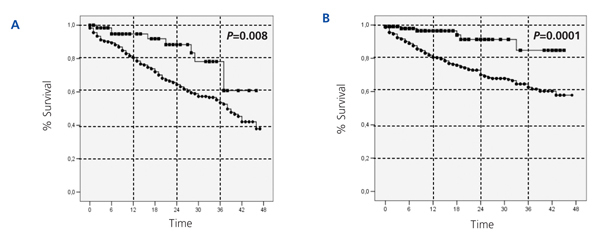
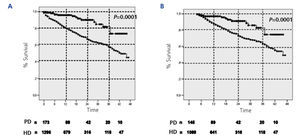
The cumulative mortality rate from the ITT analysis was 27.1% in the HD group and 8.7% in the PD group (P<.0001). Similarly, in the analysis based on survival after the 90th day of dialysis treatment, the cumulative mortality rate was 13.5% in the HD group and 5% in the PD group (P<.0001). Figure 1 displays the comparative Kaplan-Meier survival curves for the global sample from day 0 (Figure 1A), and from day 90 (Figure 1B). In greater detail, the cumulative probability of survival by ITT for PD and HD was 96.6% vs 89% at 6 months (P<.001), 96% vs 80% at 12 months (P<.001), 90% vs 65% at 24 months (P<.001), 82% vs 58% at 36 months (P<.001), and 73% vs 45% at 46 months (P<.001). The same analysis starting at day 90 yielded very similar results.
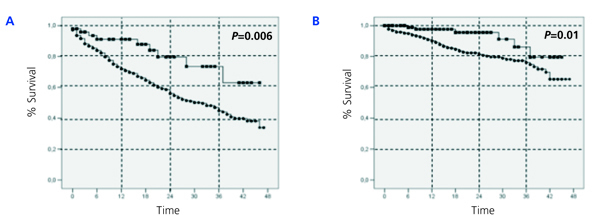
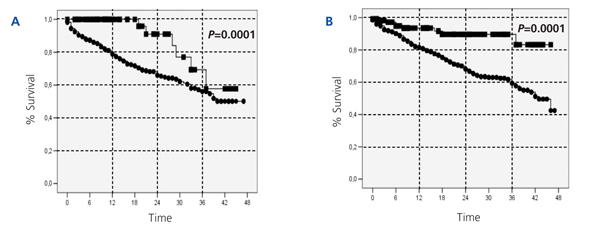
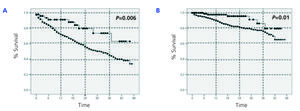
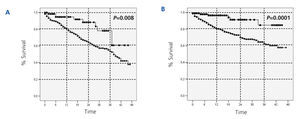
With regards to the analysis by subgroups, survival was surprisingly higher in patients on PD than those on HD, both in patients older and younger than 65 years, in both diabetics and non-diabetics, and in both women and men (Figure 2, Figure 3, and Figure 4). In the score 1 group (the group with the highest probability of choosing HD, n=430), patient mortality was 40% for patients on HD (168/412), and 22.2% for those on PD (4/18) (P=.08). For the score 4 group (the group with the highest probability of choosing PD, n=380), mortality was 12.3% for HD patients (38/308), and 5.6% for PD patients (4/72) (P=.06).
The primary causes of death were: cardiovascular (41%), infectious (19%), neoplastic (7.7%), and gastrointestinal or hepatic (3%). We observed no differences with regard to the distribution of causes of death between the two patient groups. 26.6% of the patients on PD received transplants, and 14% were transferred to HD. Of these, mortality was not different from those that did not switch treatments (8.8% vs 8.7%). In the HD group, 10.2% of patients received transplants, and only 1.7% of patients (n=22) were transferred to PD, with somewhat lower mortality observed in these patients than in those that did not switch (9.1% vs 27.4%, P=.055).
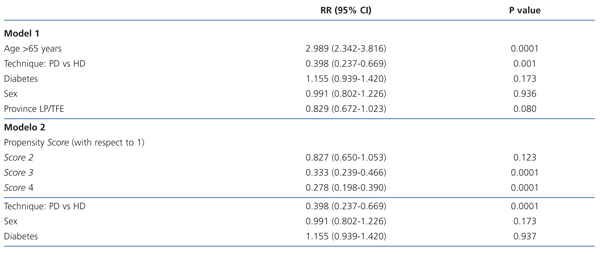
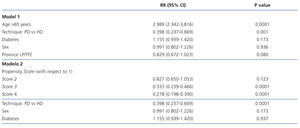
In the ITT analysis, the mortality risk adjusted by model 1 for age, sex, patient’s province of origin, and diabetes was 61% lower for the PD group than for HD (0.398; 95% CI: 0.237-0.669; P=.001; Table 3). Age also stood out as a significant risk factor for mortality. In model 2 (Table 3), we adjusted the model using the following independent variables: sex, diabetes, and propensity score. In this model, the Cox-adjusted mortality risk was also 61% lower for PD patients than for those on HD (RR: 0.398; 95% CI: 0.237-0.669; P=.0001), and the mortality risk for patients in the propensity score 4 group (those with the highest probability of choosing PD) was 63% lower than for those in group 1 (the highest probability of choosing HD) (RR: 0.278; 95% CI: 0.198-0.390; P=.0001).
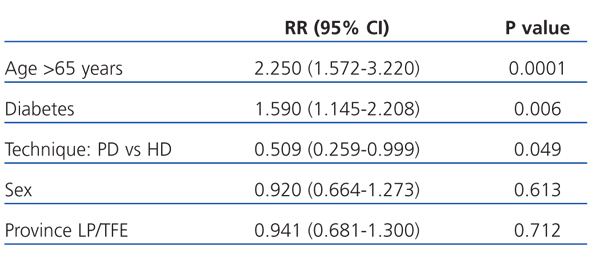
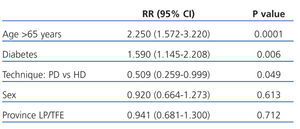
Broken up into the first 3 years of follow-up, the relative risk of mortality for patients on PD compared to HD in the first year was 0.334 (95% CI: 0.136-0.818; P=.016). After the first year of survival on dialysis, the predictors for mortality were age, diabetes, and dialysis technique (Table 4). After the second year, only age remained a risk factor for mortality (2.785; 95% CI: 1.525-5.086; P=.001), and no differences were observed between the two different dialysis techniques.
DISCUSSION
Our study has shown that PD holds significant advantages in terms of medium-term survival over HD in the Canary Islands.
Several studies have reviewed comparative survival between patients on PD and HD, with varying and sometimes contradictory results, probably due to the heterogeneity of confounding factors that may affect the prognosis of these patients,3,10-22,33-41 such as: initial comorbidity, the experience at the dialysis centre, a programmed or urgent start to dialysis, complications in peritoneal and vascular access points, the length of time on dialysis, and the different study designs, samples, and statistical methodologies used for analysing the results. In theory, prospective cohort studies are the best type of study design for studying the relationship between two variables (treatment type and prognosis) through time. However, few studies of this type have been published that analyse comparative survival between patients on HD and PD, since they require large sample sizes in order to achieve adequate stratification and adjustment of the study population, which generally requires a multi-centre study design.1,7 As such, observational studies covering a large sample of patients are the most useful in comparative studies that assess the differences in results between the two different dialysis techniques. In our case, we have added a propensity analysis in order to make our analysis more robust.8,10
Several different studies have demonstrated that PD yields better survival rates than HD in the first and second years of treatment, above all in younger, non-diabetic patients with lower comorbidity, whereas HD appears to produce better results in older, diabetic patients with greater comorbidity.3,10,21,40,42,54 However, these studies all refer to incident dialysis patients from almost 10 years ago, specifically in the 90’s and early 2000’s, when PD was still underdeveloped and recent technical improvements had yet to be introduced. Indeed, a recent study published in the United States by Mehrotra et al, with almost 700 000 incident dialysis patients, compared long-term mortality between HD and PD and reported that survival was similar between both techniques, both in the global sample and when adjusted by group according to age, diabetes, and comorbidity.5 They also observed that the survival of patients on PD has improved spectacularly in recent years, specifically in the cohort of patients from 2002 to 2004 with respect to previous years.
When researching comparative survival between dialysis techniques, it is also important to perform an analysis of survival in different time periods. Several different large-scale observational studies2,42 report that the relative mortality risk of PD as compared to HD is unstable through time, such that the survival advantage of PD decreases through time on dialysis, with advanced age, and with the presence of diabetes. However, in our study, only age turned out to be a risk factor for mortality in our patients between the second and third or fourth year of treatment, while before 2 years, HD (and not PD), along with diabetes mellitus and age were significant risk factors for mortality. This observation was supported by the Yeates study35 in 2008 (abstract), which concluded that the initial advantages of PD were maintained for a longer period of time, and even showed that no statistically significant differences existed in favour of HD even at the end of the follow-up period.1,35 Recently, a publication from the EDTA records with data from the United Kingdom, Austria, Spain, Italy, and Norway, and adjusted for comorbidity (age, primary kidney disease, sex, and nationality) showed that no significant differences existed between the two different types of dialysis treatment.9,38
One could reasonably argue that pre-dialysis education, which was widely extended throughout the Canary Islands in recent years, could have influenced these improved survival results for PD, but it would be logical then that the positive impact on survival rates due to the advanced chronic kidney disease (ACKD) education would benefit both techniques, not just PD. Furthermore, improved survival in HD patients is known to be one of the greatest impacts of ACKD education, since it notably improves the number of patients that starts treatment with a developed vascular access point.48 However, ACKD education could influence the patients’ choice of PD over HD in a greater number of cases.49
Another hypothesis that could explain the results observed in favour of PD could be the development in the last decade of new PD solutions that attempt to reduce bio-incompatability and improve clinical results in PD patients. In animal models, long-term exposure to biocompatible solutions causes reduced expression of vascular endothelial growth factor, an increase in mesothelial cell mass, and a reduction in proinflammatory markers, microvascular proliferation and sub-mesothelial fibrosis compared to conventional glucose solutions.23-32,43-47 The clinical benefits related to the greater biocompatibility of these solutions have been pointed out on several occasions, and include reduced pain and abdominal discomfort during infusion, reduced incidence and duration of peritonitis, reduced formation of final products from advanced glycosylation, and increased patient survival when compared to conventional solutions.23-27,43-47 However, to our knowledge we currently still lack medium-term mortality studies comparing incident dialysis patients on HD and those on PD, using the new, more biocompatible solutions for the PD patients. Our study did not take into account the variable “type of peritoneal dialysis solution” (standard or biocompatible) for PD patients. However, since early 2006, approximately 70% of patients that started PD in the Canary Islands received treatment with biocompatible solutions, i.e., solutions with physiological pH and a bicarbonate buffer, low concentrations of glucose degradation products, amino acids, and glucose polymers. This may have influenced the positive results observed in diabetic patients. A specifically designed study could uncover the precise role of these new solutions in improving survival of patients on PD.
There are limitations to this study. In the first place, since dialysis type was not randomly assigned to each patient, direct causality cannot be inferred, and although the propensity analysis is a useful statistical tool, it is no substitute for randomisation. Secondly, the study was performed retrospectively, using the database compiled by the Canary Islands registry of renal patients, and the prognostic biomarkers and covariables were only measured at the start of dialysis treatment. Additionally, important variables were not present in the registry, the most important being comorbidity. These gaps will require further studies to research the potential benefits of different treatment components, such as biocompatible solutions in patients with high cardiovascular risk. Nevertheless, older patients and diabetic patients tend to have high associated comorbidity. In our study, even these patients had better survival rates when on PD. Thirdly, we were also unable to analyse data on the dialysis dosage, type of HD (online or standard), manual or automated PD, residual diuresis, and the start of dialysis with a central venous catheter. With respect to the latter, dependence on a central venous catheter remains high in HD patients during the first months of treatment, and its use is associated with higher rates of morbidity and mortality. Indeed, mortality of patients on HD was higher in the first 3 months of our study, and dropped from 27.1% at the start of treatment to 13.5% after three months. This could be related to the start of HD with a venous catheter instead of an arteriovenous fistula (AVF). In PD, mortality from day 0 was 8.7%, and dropped to 5% after 90 days, thus always lower than HD.
In spite of these limitations, our study analysed survival based on all data compiled from study patients, using a good Cox analysis and a propensity analysis in order to make the statistical methodology more robust.
In summary, we have observed that survival was greater for patients that started renal replacement therapy with PD as compared to HD, even in older and diabetic patients, in which we expected to observe a lower life expectancy and greater level of comorbidity. Until now, the majority of the studies indicated that, in these patients, HD technique was superior to PD.
Although this study may have clinically relevant implications for healthcare in the Canary Islands, our results must be verified by studies in other populations and with a greater sample size, as factors such as geography, race, and health systems can influence the results of a study with a similar design.
Finally, with regard to the economic costs of treatment, PD again offers advantages over HD. In a cost-effectiveness review of different types of dialysis, Arrieta indicated that the cost of HD is approximately 47 000 euros per patient per year, whereas PD is around 32 000.50 These differences in cost are substantial, and must be taken into account when deciding which technique to prescribe or when designing a dialysis centre.52-54 The study by Berger et al,53 started in January 2004, also researched this aspect of dialysis care, comparing costs of PD versus HD in incident dialysis patients with a minimum follow-up period of 1 year, and using propensity score matching between patients on PD and HD. HD patients were hospitalised more than double the number of times that PD patients, and the mean healthcare cost incurred over the 12-month follow-up period was 43 510 dollars more in HD than in PD. The study by Mendelsson et al22 assessed a far-reaching survey performed among nephrologists with regard to their patients. They observed that 78% of patients that started dialysis had no contraindications for PD, which would indicate that an optimal and affordable distribution of dialysis methods is necessary among patients that start renal replacement therapy.
CONCLUSIONS
To conclude, in this analysis we verified that PD provides advantages in terms of survival over HD, and especially, that these results continue to stand out even when the data is stratified by age, diabetes, and sex. Given that both methodologies continue to be improved year after year, periodic reviews of comparative survival rates can help inform patients regarding their decision as to which type of dialysis to receive.
Acknowledgements
The authors would like to thank the entire nephrological community that has provided data for the RERCAN. We would also like to thank Marcos Getino Melián, technician at the Dirección General de Programas Asistenciales (General Directorate of Healthcare Programmes), Canary Island Health Services, for supervising the registry data collection.
Table 1. Comparison of baseline characteristics of patients starting PD and those starting HD
Table 2. Logistical regression. Factors associated with choosing PD as the technique of first choice
Table 3. Cox proportional regression model for survival to estimate the relative risks of mortality while on PD compared to HD
Table 4. Cox proportional regression model for survival to estimate the relative risks of mortality while on PD as opposed to HD after the first year of survival
Figure 1.
Figure 2.
Figure 3.
Figure 4.