In December 2019 an outbreak of pneumonia-causing coronavirus disease (COVID-19) began in Wuhan, China.1 In a matter of weeks, the infection spread globally, was classified as a pandemic by the World Health Organisation (WHO)2 and caused health systems to collapse in many countries, with an increase in mortality associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Although no consistent scientific evidence has established a definitive antiviral treatment, management with ventilatory support has been the essential basis for survival in many patients.3 Patients with chronic kidney disease (CKD) on renal replacement therapy (RRT) have an increased risk of mortality due to coronavirus.3 Omalizumab is a humanised monoclonal antibody that lowers levels of free immunoglobulin E and prevents its binding to its FceRI receptor, thereby reducing the activation of the allergic cascade, basophils and mast cells.4
We describe the first case of COVID-19 in a haemodialysis (HD) patient treated with omalizumab. A 56-year-old woman with a history of chronic urticaria since 2013, who developed CKD secondary to rapidly progressing glomerulonephritis associated with MPO-ANCA vasculitis that was refractory to treatment (steroid pulse therapy, mycophenolate mofetil, rituximab, cyclophosphamide and plasmapheresis).The patient required he initiation of HD in April 2019. Urticarial vasculitis flares are considered a trigger for the deterioration of kidney function, so omalizumab was started. The patient did not recover kidney function, so was maintained on HD. Her maintenance treatment consisted of prednisone (12.5 mg/d) and omalizumab (300 mg/once per month [last dose 17 April 2020]).
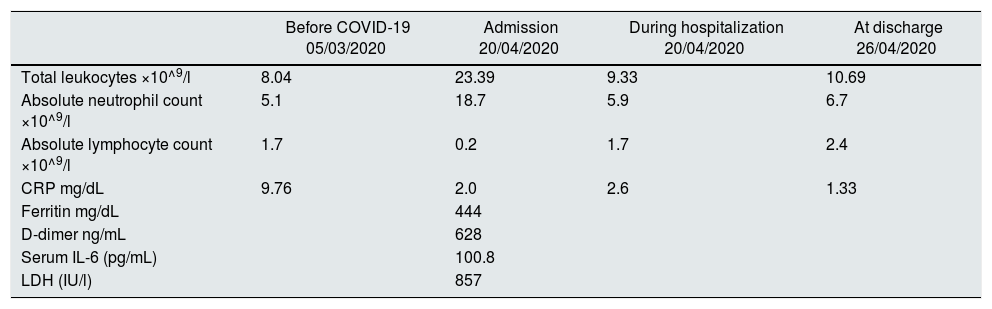
On April, the 20th of 2020, upon finishing her routine HD session, the patient was referred to the Emergency Department in our hospital with fever (38.4 C°, 48 h' evolution), nonproductive cough and non-specific abdominal pain. On admission, her arterial oxygen saturation at room air was 98% and she remained stable throughout the hospitalisation. Blood work revealed leukocytosis with neutrophilia. Acute phase reactants were significantly elevated, with d-dimer, C-reactive protein (CRP) and interleukin 6 (IL-6) of 628 ng/mL, 2 mg/dL and 100.8 pg/mL, respectively (Table 1). The chest x-ray showed no infiltrates or pleural effusion. SARS-CoV-2 infection was diagnosed by using real-time PCR of a nasopharyngeal swab. The patient received treatment with azithromycin and hydroxychloroquine for three and five days, respectively. Based on WHO criteria,2 our patient developed a mild clinical course of the disease. As an intercurrent process, she presented urinary sepsis caused by Pseudomonas aeruginosa, which was treated empirically with ceftazidime, and subsequently with amikacin after adjustment based on an antibiogram, completing two weeks of treatment in total. After one week of hospitalisation, her clinical course was satisfactory with no need for ventilatory support at any time, leukocyte levels were lower and her initial clinical picture had improved, so she was discharged with her usual immunosuppressant treatment.
Laboratory findings before infection with COVID-19, during hospitalisation and at discharge.
| Before COVID-19 05/03/2020 | Admission 20/04/2020 | During hospitalization 20/04/2020 | At discharge 26/04/2020 | |
|---|---|---|---|---|
| Total leukocytes ×10^9/l | 8.04 | 23.39 | 9.33 | 10.69 |
| Absolute neutrophil count ×10^9/l | 5.1 | 18.7 | 5.9 | 6.7 |
| Absolute lymphocyte count ×10^9/l | 1.7 | 0.2 | 1.7 | 2.4 |
| CRP mg/dL | 9.76 | 2.0 | 2.6 | 1.33 |
| Ferritin mg/dL | 444 | |||
| D-dimer ng/mL | 628 | |||
| Serum IL-6 (pg/mL) | 100.8 | |||
| LDH (IU/l) | 857 |
Recently, Henry et al. conducted a meta-analysis of four studies in 1389 COVID-19 patients to analyse the effect of CKD on the severity of COVID-19 infection. Their results suggest that CKD is a risk factor for developing a severe form of SARS-CoV-2.5 Although individually the studies were unable to conclude that CKD was a significant clinical predictor of severe COVID-19, taking together the data from the four studies analysed a significant association was found between CKD and severity of the disease.
The ongoing registry of RRT patients by the Sociedad Española de Nefrología (Spanish Nephrology Society - SEN) included 868 patients in its first publication (kidney transplants, patients in peritoneal dialysis programmes and patients in chronic haemodialysis programmes), demonstrating the severity of the disease in this cohort with a very high rate of admissions (85%) and mortality (23%).6 A single-centre observational study conducted by Goicoechea et al.7 in a population of HD patients found that the mortality rate is very high (up to 30.5%) in comparison with that observed in the general population secondary to COVID-19 infection (1.4%–8%). Interestingly, the mortality of HD patients in the two Spanish studies was almost the same as or even higher than that reported in an intensive care unit (ICU) in Italy (26%).8 Lymphopaenia and elevated lactate dehydrogenase (LDH) levels (both present in our patients) were associated with a poor prognosis.7 We believe that the mild clinical course of COVID-19 in our HD patient may be due in part to the protection conferred by treatment with omalizumab.
Conflicts of interestDr MJ Soler declares fees unrelated to the current work from NovoNordisk, Jansen, Boehringer, Eli Lilly, AstraZeneca, Esteve, FMC and Mundipharma.
Please cite this article as: Baldallo C, León Román JC, Seron D, Agraz I, Solans R, Ramos N, et al. Infección por COVID-19 en una paciente con síndrome urticarial hipocomplementémico y vasculitis ANCA MPO en hemodiálisis tratada con omalizumab. Nefrologia. 2021;41:354–355.