Dear Editor,
Sodium valproate is an antiepileptic drug also used in situations of acute mania and prophylaxis of bipolar disorder. Although acute valproate poisoning often causes a mild, self-limiting depression of the CNS, serious toxicity and death have been encountered.
Extracorporeal drug clearance is indicated for clinically severe overdose that does not improve with other, less invasive measures. Theoretically, the low molecular weight (144 Daltons) and low volume of distribution suggest a benefit from extracorporeal therapy, even if valproate has a high degree of binding to serum proteins. However, at values above 100µg/ml the protein binding sites become saturated and there is an increase of levels of free drug that can be quickly filtered using a dialysis membrane. It also has the benefit of reversing the metabolic acidosis associated with toxic levels of valproate. A limited number of cases in the literature describe the use of haemodialysis or haemoperfusion in the treatment of valproate overdose, so a description of this case is quite important and relevant.
A 62 year-old female patient, with a history of depressive psychiatric disorders, suicidal thoughts and atrial fibrillation. She received outpatient treatment with sertraline, diazepam, lorazepam, warfarin and amiodarone. She was admitted to hospital emergency on 28 November 2008, through the emergency service (112), due to ingesting 60 x 500mg sodium valproate tablets (> 200mg/kg) within the previous 12 hours. She was in a coma of 3 on the Glasgow Coma Scale (GCS). Generalised hypotonia. Mydriatic pupils reactive to light. Extremities cold and cyanotic. Hypothermia. Eupneic. Hypotensive. Heart rate of 67bpm. Serum therapy and amine treatment was started.
Analytically, there was slight leukocytosis (12,720) and metabolic acidosis (pH 7.21). Electrolytes, calcium, phosphorus, kidney and liver function without alterations.
Levels of benzodiazepines in the urine were negative and blood valproate was requested.
She was taken to the intensive care unit for respiratory depression and due to elevated serum sodium valproate (> 600mg/ml). She was intubated and connected to mechanical ventilation.
Carnitine treatment was started at a dose of 1g IV every 8 hours, 60mL activated charcoal every 4 hours and continuous venovenous haemodiafiltration (CVVHDF) with blood pump 200mL/min; dialysate flow rate of 1,500mL/h; replacement fluid flow 1,500mL/h, heparin; PRISMA®.
After 48 hours and after maintaining a good urine output, diafiltration was stopped and a significant decrease in blood levels of valproate was found.
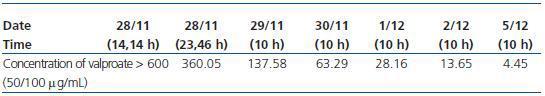
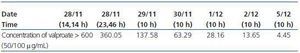
Despite having preserved kidney function, there were no complications due to the CVVHDF technique (Table 1).
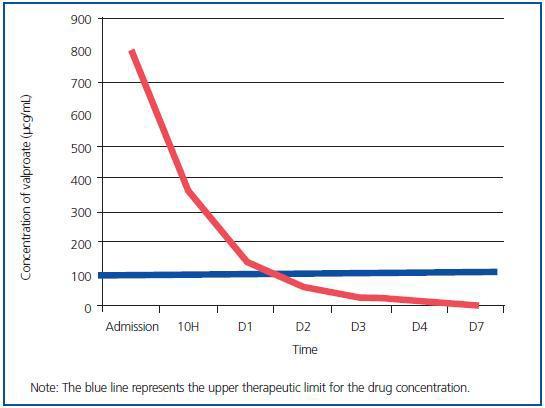
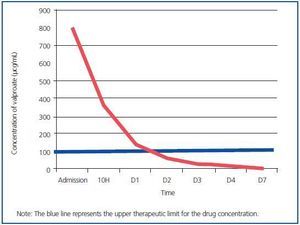
As can be seen in the table, therapeutic levels of valproate were reached after 48 hours CVVHDF. More than 99% of drug was removed from serum levels after 5 days thanks to the treatment given, which included continuous kidney clearance (Figure 1).
On the third day of admission there were clinical, analytical and radiological alterations indicative of the existence of nosocomial pneumonia. Staphylococcus simulans and Staphylococcus aureus were isolated from bronchial secretions, so antibiotic therapy with levofloxacin was initiated. After 4 days of treatment and haemodynamic and metabolic stability, she was transferred to the internal medicine service, with psychiatry support. She was discharged from the internal medicine service on 15 December 2008.
Figure 1.
Table 1. Evolution of valproate in blood