Hyperkalaemia is a common electrolyte imbalance with potentially serious short-, medium- and long-term consequences on morbidity and mortality rates and the use of national health service resources. The fact that different medical specialities can manage hyperkalaemia makes it important to have a unified approach, and the recent availability of new specific drug treatments means that the approach needs to be updated. This consensus document from the scientific societies most directly involved in the management of hyperkalaemia (Sociedad Española de Cardiología [Spanish Society of Cardiology], Sociedad Española de Endocrinología y Nutrición [Spanish Society of Endocrinology and Nutrition], Sociedad Española de Medicina Interna [Spanish Society of Internal Medicine], Sociedad Española de Medicina de Urgencias y Emergencias [Spanish Society of Accident and Emergency Medicine] and Sociedad Española de Nefrología [Spanish Society of Nephrology]) first of all reviews basic aspects of potassium balance and blood potassium. Then it goes on to focus on the concept, epidemiology, pathophysiology and diagnostic and therapeutic approaches to hyperkalaemia. The available evidence and the main published studies have been reviewed with the aim of providing a useful tool in the multidisciplinary approach to patients with hyperkalaemia.
La hiperpotasemia es una alteración electrolítica frecuente con consecuencias potencialmente graves a corto, medio y largo plazo, tanto en términos de morbilidad y mortalidad como de consumo de recursos del Sistema Nacional de Salud. El abordaje de la hiperpotasemia por diversas especialidades médicas y la reciente disponibilidad de nuevos tratamientos farmacológicos específicos hace necesaria una acción unificada y actualizada. El presente documento de consenso entre las sociedades científicas más directamente implicadas en el abordaje de la hiperpotasemia (Sociedad Española de Cardiología, Sociedad Española de Endocrinología y Nutrición, Sociedad Española de Medicina Interna, Sociedad Española de Medicina de Urgencias y Emergencias y Sociedad Española de Nefrología) repasa, en primer lugar, aspectos básicos del balance de potasio y de la potasemia, centrándose posteriormente en el concepto, epidemiología, fisiopatología, y abordaje diagnóstico y terapéutico de la hiperpotasemia. Se han revisado las evidencias y los principales estudios publicados con el objetivo de que sea una herramienta útil en el abordaje multidisciplinar del paciente con hiperpotasemia.
Hyperkalemia is a common electrolyte disturbance with potentially serious consequences in the short, medium and long term, both in terms of morbidity and mortality and in the consumption of National Health System resources. The progressive aging of the population, the increasing incidence of diabetes mellitus, chronic kidney disease (CKD) and cardiovascular diseases (CVD), with special emphasis on heart failure, as well as the increasingly common use of drugs whose adverse effects include an increase in serum potassium concentration, means that hyperkalemia is becoming more frequent in daily clinical activity.
The approach to hyperkalemia by various medical specialties and the recent availability of new specific pharmacological treatments requires unified and updated action. To meet this demand it arose the initiative to generate a consensus among the scientific societies most directly involved in the approach to hyperkalemia and which have signed this document: Spanish Society of Cardiology, Spanish Society of Endocrinology and Nutrition, Spanish Society of Internal Medicine, Spanish Society of Emergency Medicine, and Spanish Society of Nephrology. Each society has appointed delegates who have participated in the drafting of the document and are listed as authors. The final text is the result of discussion and approval by all delegates.
This consensus document first reviews basic aspects of potassium balance and potassemia, and then focuses on the concept, epidemiology, pathophysiology, and diagnostic and therapeutic approach to hyperkalemia. The evidence and major published studies have been reviewed with the aim of making it a useful tool in the multidisciplinary approach to the patient with hyperkalemia.
The basic concepts of potassium balance and potassemiaPotassium is the third most abundant mineral in humans, and the maintenance of an adequate potassium concentration, both inside and outside the cells, is essential for several functions, including the maintenance of membrane potential in excitable tissues. Multiple mechanisms maintain potassium homeostasis and potassemia within very narrow margins. Disruption of any of these mechanisms may precipitate the onset of hyper- or hypokalemia. The main factors influencing the serum level of potassium are dietary intake, transcellular transfer and urinary elimination of potassium.
Intracellular and extracellular potassium concentration homeostasisPotassium is the main intracellular cation. Potassemia is regulated by potassium entering and leaving the cell.1 Potassium from the diet is rapidly absorbed passively by the small intestine and accumulates intracellularly before being excreted in urine. Several factors modify its transcellular flux. Insulin and beta-2-adrenergic activity introduce potassium into the cell, reducing potassemia, while exercise, metabolic acidosis, hyperglycemia, hyperosmolarity and tissue destruction release potassium from cells, increasing its serum concentration. Although potassemia is usually maintained in the normal range despite physiological variations related to diet and physical activity, even during prolonged fasting (low insulin levels) or extreme physical exercise, these oscillations could become clinically relevant in patients that develop hypo- or hyperkalemia.2–4 Finally, several drugs interfere with transcellular potassium transfer (Table 1).5,6
Regulation of transcellular movement of potassium.
| Factors that increase the entry of potassium into the cell and promote hypokalemia. | Factors that increase potassium outflow from the cell and promote hyperkalemia |
|---|---|
| InsulinExogenous insulinCarbohydrates | Insulin deficiencyInadequately controlled diabetesFasting |
| Beta-2 adrenergic stimulationStressBeta-adrenergic agonists (albuterol, terbutaline, dobutamine, nasal decongestants or slimming agents with pseudoephedrine or ephedrine, meat contaminated with clenbuterol) | Non-selective beta-blockersPropranolol, labetalol |
| AlkalemiaIncreased extracellular pH due to metabolic or respiratory alkalosis | Metabolic acidosisExcept lactic acidosis or ketoacidosis |
| Increase in the number of cellsAcute increase in erythropoiesis | Cell destruction or red blood cell transfusions |
| OthersAldosteroneHypothermiaIntoxications (barium, cesium, chloroquine)Antipsychotics (risperidone, quetiapine) | OthersHyperosmolality (hyperglycemia, hypernatremia, hypertonic radiocontrast, hypertonic mannitol) ExerciseDigitalis intoxicationSuccinylcholine in muscle diseaseArginine HClCalcineurin inhibitors, nicorandil |
The recommended daily intake of potassium is around 3,500 mg (90 mEq), requirements that are easily met with regular intake, given its wide distribution in multiple foods and beverages.7,8
The foods richest in potassium are fruits and vegetables, although it is also present in meat, nuts, legumes, seeds and cocoa (Table 2). Another important source of potassium is potassium salts, used as a substitute for common salt.9,10 The use of these salts may increase after a recent clinical trial demonstrated that their use decreases cardiovascular events and death from any cause in hypertensive patients with a history of stroke or age 60 years or older.11 Plant sources of potassium are also a source of dietary alkalis, which can reduce metabolic acidosis in CKD by helping to maintain potassium within the cell.
Foods high in potassium. With green background, some examples of fruits with a lower potassium content.
| Potassium content (mg/100 grams of raw product) | |
|---|---|
| Vegetables | |
| Soybean | 1.730 |
| Wheat germ | 871 |
| Potato | 525 |
| Spinach | 380 |
| Chard | 378 |
| Eggplant | 262 |
| Tomato | 236 |
| Cauliflower | 193 |
| Beets | 190 |
| Fruits | |
| Ciruela passes | 782 |
| Dates | |
| Aguacate | 400 |
| Banana | 350 |
| Grapes | 250−320 |
| Melon | 320 |
| Apricot | 293 |
| Peach | 260 |
| Cherry | 255 |
| Pineapple | 250 |
| Orange | 200 |
| Mandarin | 160 |
| Pear | 130 |
| Watermelon | 120 |
| Apple | 99 |
| Nuts | |
| Almond | 767 |
| Pistachio | 811 |
| Hazelnut | 636 |
| Nut | 690 |
| Legumes | |
| White Beans | 1.337 |
| Garbanzo | 1.000 |
| Lentils | 463 |
| Cocoa | 712 |
| Meat | 270−490 |
| Seeds | |
| Lino | 813 |
| Pumpkin | 809 |
| Quinoa | 780 |
| Sesame | 450 |
Potassium leaves the body in a regulated manner in urine and in an unregulated manner in feces, vomit or sweat. Urinary potassium excretion is regulated at the level of the distal nephron.6,7 Aldosterone promotes potassium secretion by the principal cells of the connecting segment and cortical collecting tube, which exchange it for sodium reabsorption from the tubular lumen. This process can be inhibited, even in the presence of hyperkalemia, by drugs that decrease the levels or block the action of aldosterone, by a decrease in the sodium available in the lumen of the distal nephron, or by the inability of the principal cells to reabsorb sodium (Table 3). Conversely, increased sodium availability in the distal nephron (e.g., by diuretics that inhibit sodium reabsorption in more proximal segments of the nephron) favors sodium reabsorption in exchange for potassium, which is secreted into the urine, even in the presence of hypokalemia. Polyuria interferes with the kidney's ability to retain potassium, since there is a reduced urinary potassium concentration, and the high urine volume is accompanied by increased urinary potassium loss (Table 3). Excessive unregulated loss of potassium in digestive secretions or sweat may promote hypokalemia.
Regulation of renal excretion of potassium.
| Factors that increase urinary potassium excretion and promote hypokalemia | Factors that decrease urinary potassium excretion and promote hyperkalemia |
|---|---|
| Mineralocorticoid hyperactivityHyperaldosteronismLicoriceOther drugs: itraconazole, posaconazole, abirateroneGenetic origin | Mineralocorticoid hypoactivity Decrease of aldosteroneDrugs (RAS blockade, NSAIDs, calcineurin inhibitors, heparin) Genetic (e.g. Gordon)Decreased response to aldosteronePotassium-sparing diureticsDistal renal tubular acidosis |
| Proximal tubule diuretics, loop diuretics and thiazidesIncreased sodium in distal nephron lumen | Decreased distal flow of water and sodiumVolume depletion |
| Non-resorbable anions in distal tubuleBicarbonate (vomiting, proximal tubular acidosis)Beta-hydroxybutyrate (diabetic ketoacidosis, hypocaloric diet)Hypurate (use of toluene; glue sniffing)Penicillin derivative | Acute or chronic kidney disease |
| Polyuria | |
| OthersTubulopathies (salt-losing: Bartter and Gitelman / Others: Liddle, renal tubular acidosis)HypomagnesemiaAmphotericin B |
There is no universally accepted definition of hyperkalemia. Laboratories provide a range of normality based on the distribution of potassemia in the general population, but it would be desirable to evolve to a definition of hyperkalemia based on health risk. The recent Kidney Disease Improving Global Outcomes (KDIGO) conference defined hyperkalemia as potassemia above normal, taking into account that serum levels of potassium are 0.1−0.7 mEq/L higher than tha plasma levels, so laboratories should indicate in which fluid it was measured and what the normality values are for each fluid.12 Serum is usually used n routine analyses, whereas plasma is commonly used in emergency department analyses because of the faster sample preparation time. In any case, KDIGO suggests classifying acute hyperkalemia as mild, moderate or severe depending on potassium levels and electrocardiographic impact, using the respective cut-off points at 5.0 mEq/L (or the upper limit of laboratory normal), 6.0 mEq/L and 6.5 mEq/L (Table 4). Acute hyperkalemia is considered when it was not previously known, but there is no consensus on the magnitude, duration and frequency of elevated potassium values that define chronic hyperkalemia.
Definition and severity of acute hyperkalemia according to KDIGO expert opinion.
| ECG changes | Potassemia (mEq/L) | ||
|---|---|---|---|
| 5.0 (or upper limit of normal) to 5.9 | 6.0−6.4 | ≥6.5 | |
| Yes | Moderate | Serious | Serious |
| No | Slight | Moderate | Serious |
ECG: electrocardiogram.
Based on reference12.
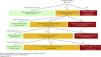
In clinical laboratories, the prevalence of hyperkalemia, defined as potassemia greater than 5.0 mEq/L, is around 3−4% in unselected populations, but increases greatly in populations with predisposing factors. The highest prevalence is observed in hemodialysis patients. A recent study in patients with CKD and glomerular filtration rate (GFR) below 60 mL/min/1.73 m2 illustrates the natural history of hyperkalemia as a recurrent phenomenon, with progressively increasing risk of recurrence and progressively shorter time to recurrence (Fig. 1).13 The incidence of hyperkalemia with potassemia above 5.0 mEq/L was 9, 18, 31 and 42% in one year, in people with GFR of 45–59, 30–44, 15–29 and <15 mL/min/1.73 m2, respectively.13 In this regard, the inability of the kidney to adequately eliminate potassium from the diet is the main predisposing factor, either because there is CKD or other conditions that limit renal excretion of potassium, or because of taking drugs that interfere with its renal elimination, of which the main ones are renin-angiotensin system (RAS) blockers, potassium-sparing diuretics and mineralocorticoid receptor antagonists (MRA) (Table 5). Hyperkalemia is especially frequent if several of these factors are combined.
Incidence of first episode of hyperkalemia and recurrence of hyperkalemia in patients with CKD (adapted from Thomsen RW, et al.13).
Conditions and drugs associated with hyperkalemia.
| Conditions |
| Chronic kidney disease: low glomerular filtration rate and/or high proteinuria. |
| Diabetes mellitus |
| Cardiovascular disease |
| Congestive heart failure |
| Coronary artery disease |
| Peripheral arterial disease |
| Malignancy |
| Anemia |
| Hyperlipidemia |
| Metabolic acidosis (non-organic) |
| Reduced secretion of or response to aldosterone |
| Gota |
| Ureteroyojejunostomy |
| Disintegration of cells or tissues (e.g., rhabdomyolysis, hemolysis) |
| Exercise |
| Drugs |
| Renin-angiotensin-aldosterone system inhibitors |
| Potassium-sparing diuretics |
| β-blockers |
| Non-steroidal anti-inflammatory drugs |
| Potassium supplements or salts containing potassium chloride |
| Calcineurin inhibitors (cyclosporine, tacrolimus) |
| Mannitol |
| Heparin |
| Digital |
| Penicillin G |
| Succinylcholine |
| Octreotide |
| Diazoxide |
| Minoxidil |
| Volatile anesthetics (e.g., isoflurane) |
Hyperkalemia is associated with poorer health outcomes. Severe acute hyperkalemia can be lethal, and hyperkalemia is associated with an increased risk of hospitalizations (including increased cardiac events, ventricular arrhythmias, cardiac arrest, and intensive care) and death over the next six months, ranging from two to five times that observed in controls, even in populations in which one would assume a higher tolerance to hyperkalemia, such as patients with CKD.12 The association between hyperkalemia and events could have several causes, including causality by itself (for example, malignant ventricular arrhythmias), or related causes such as suboptimal use of drugs with recognized cardiovascular and renal benefits, such as RAS blockers or MRAs, the conjunction of comorbidities or even the prescription of potassium-poor diets that are not very heart-healthy.
Diagnostic approach to hyperkalemiaIt has two aspects:
Diagnosing hyperkalemia and its severity. The criteria for classifying the severity of hyperkalemia, according to KDIGO, are detailed in the previous section12 (Table 4). Severe hyperkalemia or hyperkalemia with electrocardiographic repercussions is an emergency that requires immediate treatment.14 Other situations that can also be considered as a hyperkalemic emergency are the presence of clinical symptoms (especially muscle weakness or paralysis), severe hyperkalemia (potassemia >6.5 mEq/L), especially if there is gastrointestinal bleeding or tissue destruction (which favors continued uncontrolled potassium supply to the extracellular space), even in the absence of signs or symptoms, and also some cases of less severe hyperkalemia (>5.5 mEq/L) that is expected to worsen in the short term due to situations such as renal failure, tissue destruction, presence of metabolic or respiratory acidosis, or continued absorption of potassium at the digestive level due to gastrointestinal bleeding.15
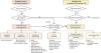
Etiologic diagnosis of hyperkalemia (Fig. 2), based on assessment of renal function, history of risk factors for renal dysfunction, drug use, urine output, blood pressure, and hydration status. In the absence of another risk factor, the amount of dietary potassium is a very rare cause of hyperkalemia.
Therapeutic algorithm for hyperkalemia. Two clinical situations are distinguished in which hyperkalemia can be observed and require acute treatment and chronic treatment, respectively; although in the absence of a clear triggering factor that is transient, all severe hyperkalemia requires chronic measures to prevent further episodes. These chronic measures should prioritize maintaining or increasing cardio-nephroprotection whenever possible.
SZC: sodium zirconium cyclosilicate; CKD: chronic kidney disease; HK: hyperkalemia; ECG: electrocardiogram; MRA: mineralocorticoid receptor antagonist; RAS: renin-angiotensin system; SGLT2: sodium-glucose cotransporter-2.
Pseudohyperkalemia should be ruled out, where hyperkalemia is due to inadequate sampling (excessive muscle contraction during venipuncture, contamination of the sample with solutions or parenteral nutrition) or outflow of potassium from cells after sampling, such as hemolysis when there is a large number of blood cells (erythrocytosis, leukocytosis, thrombocytosis) or in individuals with certain genetic variants.16 Pseudohyperkalemia should be suspected when there is no apparent cause for the hyperkalemia and no clinical or electrocardiographic repercussions.
An acute increase in potassemia may be due to redistribution of potassium by release of intracellular potassium, the cause of which is usually identified by clinical history, such as tumor lysis syndrome, metabolic acidosis or diabetic ketoacidosis.17 If hyperkalemia is persistent, it is probably due to a reduction in urinary potassium excretion secondary to an inappropriate response to aldosterone (due to reduced secretion or resistance), to acute or chronic renal disease, or to a decrease in effective circulating volume as occurs in volume depletion, heart failure or cirrhosis.
In persistent hyperkalemia without renal failure, urine electrolyte analysis can help to determine the cause. Urinary potassium excretion in 24-h urine is conditioned by dietary potassium intake, which alone does not usually lead to hyperkalemia in healthy individuals.18 A low urine sodium determination points to a decrease in effective circulating volume. The degree of aldosterone activity could be estimated from the concentration of potassium in the collecting tubule. For years it has been used the transtubular potassium gradient (TTKG):
A TTKG < 6 in the presence of hyperkalemia would indicate an insufficient response to aldosterone or hypoaldosteronism, which should be assessed by the TTKG response to mineralocorticoid administration.19 However, the authors of the equation later indicated that the theoretical postulates on which it is based may not be valid, as it does not consider the effect of distal tubular reabsorption of urea on potassium excretion.20 In any case, if hyperkalemia is persistent and no cause is found, a possible hypoaldosteronism should be studied.
Treatment of hyperkalemiaAcute treatment of severe hyperkalemiaTreatment aims to avoid the acute lethality of hyperkalemia and to decrease potassemia by three types of maneuvers15: (1) antagonizing the effects of potassium on the cell membrane; (2) bringing potassium into the cells; (3) taking potassium out of the body (Box 1Table 6). Only the last two reduce potassemia.
Acute treatment of severe hyperkalemia.
| 1). Antagonize the effects of potassium on the cell membrane: calcium gluconate or calcium chloride. |
| 2). Introducing potassium into the cells |
| a) Insulin (and glucose). |
| b) Beta2-adrenergic agonists. |
| c) Consider sodium bicarbonate if metabolic acidosis is present. |
| 3). Potassium removal from the body |
| a) Increase urinary elimination. Consider loop diuretics and thiazides, especially if hypervolemia. |
| b) Increase GI elimination. Sodium zirconium cyclosilicate (SZC). |
| c) Hemodialyze if the patient is on chronic hemodialysis program and consider hemodialysis if severe renal failure even if the patient is not on chronic hemodialysis program. |
Acute treatment of severe hyperkalemia.
| Antagonize the effects of potassium on the cell membrane. | Introducing potassium into the cells | Removing potassium from the body | |
|---|---|---|---|
| Treatment | Calcium gluconate or calcium chloride | Insulin (and glucose)beta-2-adrenergic agonists (added to insulin)Sodium bicarbonate (in case of metabolic acidosis) | Increase urinary eliminationIncrease GI eliminationHemodialysis |
| Onset of effect | 1−2 min | 10−20 min(>60−120 min for bicarbonate) | Variable, in general>60 min.Hemodialysis: 10−20 min. |
| Reduces potassemia | No | Yes | Yes |
| Risk of early recurrence of hyperkalemia | Not applicable, although there is a risk of early recurrence of the negative impact of hyperkalemia on the cardiac cell membrane. | Higha | low |
Calcium antagonizes the effect of potassium on the cardiac cell membrane. The onset of action of calcium is a few minutes, but lasts only 30–60 min, which makes it necessary to use it in combination with other strategies. It is available in two preparations: calcium gluconate and calcium chloride. Calcium gluconate 1,000 mg i.v. administered in two to three minutes with cardiac monitoring is preferred for convenience of use and less damaging effect on the veins if it is misplaced. If the emergency persists, calcium administration can be repeated every 30−60 min if there is no increase in calcemia.
Introducing potassium into the cellsThe administration of insulin and/or beta-2-adrenergic agonists and/or sodium bicarbonate (the latter only if there is metabolic acidosis) favors the entry of potassium into the cell.
Insulin activates the sodium-potassium-ATPase (Na/K-ATPase) exchange pump in the cell membrane, especially in the muscle cell. It is the most effective measure. Its effect starts in 10−20 min and lasts up to six hours. Insulin is generally administered with glucose to prevent hypoglycemia or alone if blood glucose is greater than 250 mg/dL. One of the most widespread guidelines is the intravenous administration of 10 units of insulin in 500 mL of 10% glucose saline over one hour. The aim is to maintain the effect of the insulin and avoid the onset of hypoglycemia.
Beta-2-adrenergic agonists also activate the Na/K-ATPase pump. They are effective both inhaled and intravenously and are indicated as an adjunct to insulin administration. Salbutamol is usually used, 10−20 mg in 10 min if inhaled (a much higher dose than that administered in the treatment of bronchospasm), or 0.5 mg in 100 mL of 5% glucose in 15 min if intravenous. Its action starts in 10 min if inhaled and 15 min if intravenous, lasting three hours. It can produce tachycardia. The combination of insulin and beta-2-adrenergic agonists has an additive effect on the reduction of potassemia, making it more effective than their separate use.
Sodium bicarbonate is added to the previous measures only if there is metabolic acidosis. It is not considered a fundamental measure as it has much less effect, and more delayed, than the other maneuvers.
Removing potassium from the bodyIt can be achieved in three ways: increased urinary elimination, increased digestive elimination or by dialysis.
- •
Increase the urinary excretion. Only loop diuretics have an onset and magnitude of action that allows them to be useful in the acute treatment of hyperkalemia in patients with normal or slightly impaired renal function, but they may not be effective if renal insufficiency is severe. Concomitant administration of loop or thiazide diuretics could have an adjuvant effect, especially in patients with hypervolemia.21
- •
Increase gastrointestinal elimination. There are three options:
- 1
Ion exchange resins such as sodium or calcium polystyrene sulfonate. Their use in situations of acute hyperkalemia is limited, given their slow onset of action and the absence of clinical trials that have demonstrated efficacy. In a placebo-controlled clinical trial, the association of resinsodium with cathartics did not reduce potassemia more than placebo in the following 12 h.22
- 2
Patiromer. It is a non-absorbable polymer containing a calcium-sorbitol complex. It binds potassium in the colon in exchange for calcium, having also the capacity to bind magnesium. It thus reduces the concentration of free potassium in the intestinal lumen and increases its fecal excretion. There are three authorized presentations in the form of 8.4, 16.8 and 25.2 g powder for oral suspension. REDUCE was a single-center, open-label pilot study, which randomized 43 hemodialysis patients with potassemia ≥6.0 mEq/L to treatment as usual with or without (non-placebo control group) the addition of 25.2 g patiromer, with 30 patients being finally evaluated23 (NCT02933450). The primary endpoint was potassemia at 6 h, with no differences between groups: patiromer 5.8 mEq/L (95% confidence interval [95% CI] 5.4–6.1 mEq/L) and control 6.3 mEq/L (95% CI, 6.0–6.6 mEq/L). However, at 2 h, potassemia was lower in the patiromer group than in the control group: 5.9 mEq/L (95% CI, 5.6–6.1 mEq/L) vs. 6.5 mEq/L (95% CI, 6.2–6.7 mEq/L), the baseline potassium concentration being 6.4 ± 0.5 and 6.7 ± 0.5 mEq/L, respectively. The poor efficacy of "treatment as usual" is explained by the fact that it only consisted of insulin (33% of patients) or beta-adrenergics before 2 h in less than half of the patients, whereas in that period the patients randomized to patiromer had also received insulin (40%) or beta-adrenergics. That is, at 2 h patiromer was compared with no intervention in more than half of the patients.
- 3
Sodium zirconium cyclosilicate (SZC). It is a crystalline, non-polymeric, non-absorbable potassium binder. The ENERGIZE study (NCT03337477)24 was a multicenter, double-blind, placebo-controlled, Phase 2 clinical trial evaluating the efficacy of SZC in the emergency treatment of hyperkalemia, added to glucose and insulin, with the primary endpoint being the change in potassemia at 4 h. Patients aged ≥18 years admitted to an Emergency Department and with a potassemia ≥5.8 mEq/L measured using an i-STAT rapid analyzer (Abbott Point of Care, Inc. Chicago, IL, USA), confirmed by a simultaneous determination of serum potassium in a central laboratory, were included. Although a sample size of 132 participants was estimated to achieve adequate statistical power, only 70 patients were randomized to receive 10 g SZC (repeatable at 4 and 10 h) or placebo. Overall, 30.3% (10/33) and 43.2% (16/37) of patients randomized to SZC and placebo, respectively, discontinued treatment. The treatment completion rate was 57.6% in the SZC group and 45.9% in the placebo group. At 4 h, there was no significant difference in serum potassium reduction between the two groups: mean (least squares) covariate-adjusted change of -0.41 ± 0.11 vs. −0.27 ± 0.10 mEq/L, in SZC and placebo, respectively (difference −0.13 mEq/L; 95% CI, −0.44 to 0.17). Approximately half of the patients receiving treatment in each group (57.6% in the SZC group and 45.9% in the placebo group) did not have a central measurement of serum potassium at 4 h, using the value obtained with the i-STAT adjusted to correct the difference between the two methods. Six patients in the SZC group (18.2%) and 10 (27%) in the placebo group lacked serum potassium determination by both methods, the data being imputed using the last determination made up to and including 4 h. The authors recognize as an additional limitation the potential differences in therapeutic strategies between the different centers in relation to the use of rescue therapies. In the assessment of potassium level, at 2 h there was a greater reduction in potassemia in patients randomized to SZC compared to placebo: −0.72 ± 0.12 vs. −0.36 ± 0.11 mEq/L (difference −0.35 mEq/L; 95% CI, −0.68 to −0.02). Finally, in relation to safety aspects, a similar proportion of patients experienced adverse effects in both groups in the first 24 h, being thereafter more frequent in those who received SZC (24.1 vs. 9.1%). There were no differences in terms of severe adverse effects. To summarize this section, SZC is the only intervention to increase the G.I. elimination of potassium that has been evaluated in a placebo-controlled clinical trial in the acute treatment of hyperkalemia as an added strategy to those usually used to introduce potassium into the cells, demonstrating a greater decrease in potassemia at early times (two hours) than placebo (Box 2). The PLATINUM study is currently underway, a multicenter, randomized, placebo-controlled clinical trial evaluating aspects related to the use of Patiromer in the context of hyperkalemia in emergency services.
Box 2.New developments in acute treatment of severe hyperkalemia.
1) To the classical measures available for antagonizing the effects of potassium on the cell membrane, getting potassium into the cells and getting potassium out of the body (Table 11Box 1), we must add the new oral potassium binders, which can accelerate the removal of potassium from the body in the acute treatment of hyperkalemia by a non-renal route, an advantage in patients with limited urinary potassium excretion due to anuria, oliguria, renal insufficiency or sustained activity of mineralocorticoid receptor antagonists. 2) Specifically, in patients with hyperkalemia, sodium zirconium cyclosilicate (SZC) decreased potassemia at 2 h more when added to glucose and insulin than placebo, with glucose and insulin in a multicenter, double-blind, placebo-controlled clinical trial.
- 1
- •
Hemodialysis. In a hyperkalemic emergency, especially in patients on chronic dialysis programs, hemodialysis is the treatment of choice,25 since it is the fastest and most efficient strategy to reduce potassemia, and should be applied as soon as possible, although the use of hemodialysis does not exclude the application of the previously mentioned measures. In patients with advanced CKD who are not yet in dialysis programs, hemodialysis should be considered, for which it will be necessary to have an adequate vascular access for dialysis. In general, and depending on the baseline potassemia concentration, the alterations associated with hyperkalemia are resolved within the first 30−60 min of hemodialysis.
Except when there is a clear transient and isolated cause that triggers the episode of hyperkalemia; in general, patients with severe hyperkalemia are at a high risk of recurrent hyperkalemia, this is due to the chronicity of the usual predisposing factors (CKD, heart failure, diabetes and their treatments) and it requires chronic intervention. There have been recent developments in this field (Box 3) that have changed the therapeutic approach to this complication (Box 4).
Recent developments in the chronic treatment of hyperkalemia.
| 1) Prescription of SGLT2 inhibitors can increase renal and cardiovascular protection, while reducing the risk of hyperkalemia in predisposed patients with type 2 diabetes or chronic kidney disease or heart failure. |
| 2) Sodium zirconium cyclosilicate (SZC) is safe and effective in the long-term treatment of hyperkalemia and helps correct metabolic acidosis. |
| 3) Patiromer is safe and effective in the long-term treatment of hyperkalemia. |
Chronic treatment of hyperkalemia.
| 1) Correct amendable factors: Decrease excessive potassium intake, review medication, correct metabolic acidosis |
| 2) Maintain/increase cardioprotective and nephroprotective treatment. |
| a) Attempt to maintain RAS blockade and MRA, if indicated. |
| b) Consider adding diuretics (in case of arterial hypertension, heart failure, volume overload) and/or SGLT2 inhibitors (in case of heart failure, type 2 diabetes, chronic kidney disease) if indicated to improve cardiac and renal protection while reducing the risk of hyperkalemia. |
| 3) Removing potassium from the body: intestinal potassium uptakes |
| a) Sodium zirconium cyclosilicate (SZC) |
| b) Patiromer |
| 4) Calcium polystyrenesulfonate |
Reducing the potassium content of the diet is a virtually universal measure in the management of hyperkalemia, although most clinical practice guideline recommendations for the dietary management of hyperkalemia are based on expert opinion, given the limited evidence and biases of the available studies.26 A recent meta-analysis revealed that dietary potassium restriction with the aim of reducing potassemia presents very low quality evidence, and it cannot be concluded that it decreases the risk of death or slow down the progression of CKD.27
Reducing dietary potassium load should be integrated with the other nutritional goals of the CKD patient (adequate protein intake, high fiber intake and healthy cardiovascular dietary pattern) through the following strategies28:
Nutritional education to learn about the foods with the highest potassium content, recommendations on the diet required to maintain a good nutritional status in CKD, and indications on the classification of foods according to the amount of potassium in relation to fiber. In this sense, patients should be trained to learn how to interpret the nutritional cards and food labeling, so that they can choose the most appropriate foods for their clinical situation.
Use of appropriate culinary techniques for food preparation that result in lower potassium content, such as cooking in water, pressure cooker or microwave for all foods, and prolonged soaking in water of root, tuber, leafy and cruciferous vegetables.29
Detection of hidden sources of potassium, such as low-sodium salt substitutes (no evidence in patients at risk of hyperkalemia), and certain food additives, used in up to 40% of processed products (mainly meat products, breaded products, soft drinks, ready-to-eat products and cereal products).30 The most frequently used food additives containing potassium are E202, E252, E340, E450, E452, E508 and E950.31 It is important that the patient identifies them by inspection of the nutrition labeling and restricts their consumption.
Potassium-rich foods that should generally be avoided to reduce the risk of hyperkalemia are listed in Table 2, which also includes some examples of low-potassium alternatives, duly indicated.
Compliance with a low potassium diet is not easy, given barriers such as fragmented dietary advice, contraindication to healthy eating patterns, and potential changes in appetite and taste.32 Patient-centered education strategies, motivation for transplantation or delayed dialysis, and visualization of accommodation to restrictions often facilitate adaptation to these dietary guidelines.33
Promoting the entry of potassium into cellsUnder physiological conditions, the factors that most increase potassium uptake by cells, as they stimulate Na-K-ATPase, are insulin, beta-adrenergic stimulation and, to a lesser extent, aldosterone34–37 (Table 1). On a chronic basis, adequate control of diabetes and correction of metabolic acidosis can help treat hyperkalemia, especially in CKD, which is characterized by the development of metabolic acidosis.38,39 The KDIGO guidelines suggest treatment with oral sodium bicarbonate supplementation unless contraindicated, when serum bicarbonate concentration is less than 22 mEq/L, to maintain serum bicarbonate in the normal range, regardless of potassemia.40 This approach prevents metabolic acidosis from releasing potassium from inside the cell to the extracellular space, which favors its urinary elimination.41 In a recent multicenter observational study of more than 500 non-dialysis CKD patients (66.7% with category G3b-G5), patients with incident or persistent hyperkalemia over 12 months were characterized by lower serum bicarbonate concentration, while the prescription of sodium bicarbonate was associated with a reduction in potassemia.42 Likewise, clinical trial results show that SZC corrects metabolic acidosis in CKD patients, decreasing the percentage of patients with a serum bicarbonate <22 mEq/L after 29 days of treatment from 39.4% to 17.2%.43 However, there are no clinical trials specifically designed to evaluate the effect of sodium bicarbonate on serum potassium levels or on the incidence of hyperkalemia, the existing information being derived from secondary or exploratory objectives44–50 (Table 7).
Randomized clinical trials with oral bicarbonate supplementation in patients with chronic kidney disease not on dialysis with serum potassium results.
| StudyRef / Year | Patientsrandomized/FGe | Primary objective | Duration | Groups and dosesbicarbonate | ChangePotassemiaa |
|---|---|---|---|---|---|
| So-Bic Study44/2019 | N = 35/15−60 ml/min/1,73 m2 | Propensity to calcification inserum | 4 weeks | Intervention: 45 mg/kg/dayRescue: 15 mg/kg/day | 0.1 mEq/L0.1 mEq/L |
| UBI Study45/2019 | N = 795/30−59ml/min/1.73 m2 | Time to double serum creatinine | 36 months | Control1 mEq/kg/day | No changeNo change |
| BASE Pilot Trial46/2020 | N = 190/20−59ml/min/1.73 m2 | Adherence and safety | 28 weeks | ControlHigh dose: 0.8 mEq/kg/dayLow dose: 0.5mEq/kg/day | No change−0.1 mEq/LNo change |
| Raphael et al.47/2020 | N = 74/15−89 ml/min/1,73 m2 | Renal fibrosis markers | 24 weeks | Control0.5 mEq/kg/day | No change−0.1 mEq/L |
| BICARB RCT48/2020 | N = 300 / <30 ml/min/1.73 m2 | Physical performance | 24 months | Control1.8 g/day | −0.1 mEq/L−0.1 mEq/L |
| Melamed et al.49/2020 | N = 149/15−60ml/min/1.73 m2 | Muscle function and bone mineral density | 24 months | Control1.9 or 2.6 g/day | −0.1 mEq/L−0.1 mEq/L |
| Bovée et al.50/2021 | N = 45/15−30 ml/min/1.73 m2 | Urinary renin | 4 weeks | Control3 g/day | No change−0.1 mEq/L |
Thiazides and loop diuretics increase urinary potassium excretion and can cause hypokalemia when used for other indications. They are not usually used for the sole purpose of treating chronic hyperkalemia, as they can cause volume depletion that can even lead to acute renal failure and also water and electrolyte disturbances (e.g., thiazides can cause hyponatremia). However, they can be used in cases of chronic hyperkalemia when an additional benefit is expected from their use, such as better control of arterial hypertension, heart failure and even a greater decrease in albuminuria, especially in patients with these comorbidities in whom the combination of diuretics with RAS blockade or MRA allows treatment with the latter to be maintained.51–54
Sodium-glucose cotransporter type 2 (iSGLT2) inhibitors also protect against hyperkalemia in patients with RAS blockade, in addition to improving renal and cardiovascular prognosis.55 In a recent meta-analysis of six clinical trials involving 49,875 patients with type 2 diabetes and high cardiovascular risk or CKD, iSGLT2s decreased the risk of severe hyperkalemia (potassemia ≥6.0 mEq/L) by16% without increasing the risk of hypokalemia, with a consistent effect in all subgroups, including patients treated with MRA.54,56 The mechanism is not completely clarified, but probably depends, at least in part, on increased sodium delivery to the distal nephron lumen, where it is reabsorbed in an exchange for potassium, which is eliminated in urine. It would therefore be a mechanism shared with thiazides and loop diuretics.
Limiting intestinal absorption and increasing digestive excretion of potassiumThe use of drugs that bind potassium, limiting its intestinal absorption and favoring its elimination in the feces, is a key strategy in the management of chronic hyperkalemia.
Three options are currently available:
Cation exchange resins (sodium or calcium polystyrene sulfonate). They capture potassium in the intestinal lumen in exchange for the release of sodium and calcium, respectively. Although there is ample clinical experience supporting their efficacy, the scientific evidence from placebo-controlled clinical trials is very limited, as is the number of patients included in these studies and the follow-up period, especially with the concomitant use of RAS inhibitors and MRAs57–60 (Table 8), so the safety profile has not been sufficiently evaluated in prolonged treatments of more than three weeks, which limits their use for the chronic treatment of hyperkalemia in contemporary clinical practice.61 Its G.I. tolerance is poor. Frequent G.I. side effects include constipation and nausea, which limits therapeutic compliance. Other frequent side effects are hypercalcemia (calcium polystyrene sulfonate) and hypokalemia, as a consequence of the mechanism of action. Much more rarely, cases of gastrointestinal ischemia, ischemic colitis, ulceration or necrosis of the gastrointestinal tract that may lead to intestinal perforation have been reported.61,62
Clinical trials with cation-exchange resins in the treatment of hyperkalemia.
| StudyRef(Year) | Patients | Intervention | Primary objective | Result |
|---|---|---|---|---|
| Nasir et al.57(2014) | N = 97ERC G1-G4 | PSS vs. PSC | Efficacy and safety after 72 h | Average serum K reduction: 1 mEq/L with PSC and 1.5 mEq/L with PSSFewer adverse effects with PSC |
| Lepage et al.58 (2015). | N = 33CKD (eGFR <40 ml/min/1.73 m2, no dialysis) | PSS vs. Placebo | Efficacy after 7 days | Mean serum K reduction: 1.25 mEq/L with PSS vs. 0.21 mEq/L with placebo (p < 0.001 between groups). |
| Nakayama et al.59 (2018) | N = 20CKD G4-G5 not in dialysis | PSS vs. PSC | Safety: effect on bone and mineral metabolismFour weeks | PSS: Significant reduction of serum calcium and magnesium levels, and increase of PTH.PSC: PTH reduction |
| Wang et al.60 (2018) | N = 58Hemodialysis | PSC vs. Placebo | Efficiency and safetyThree weeks | Change in serum potassium level:Control: −0.1 (−0.49, 0.32) mEq/LPSC: −0.48 (−0.75, −0.16) mEq/L (p < 0.01 between groups). |
CKD: chronic kidney disease; eGFR: estimated glomerular filtration rate; PSC: calcium polystyrene sulfonate; PSS: sodium polystyrene sulfonate; PTH: parathyroid hormone.
In Spain there are two preparations of calcium polystyrene sulfonate. In one of them, Resincalcio®, the recommended dose is 15 g three or four times a day, which should be suspended in case of constipation and is contraindicated in "renal insufficiency associated with diseases such as hyperparathyroidism, multiple myeloma, sarcoidosis or metastatic carcinoma". Sorbitol (oral or rectal) is contraindicated as a laxative, since concomitant use with calcium polystyrene sulfonate may cause colon necrosis.61,63 In the other preparation, Sorbisterit®, the dosage is 20 g, one to three times a day mixed in about 150 mL of liquid (i.e. 150−450 mL/day), at least three hours before or after oral administration of other medications or up to six hours in case of gastroparesis, as may occur in diabetes.63
The poor tolerance and poor compliance observed clinically by the authors of the present mnucript in their clinical practice were recently confirmed in a crossover clinical trial versus patheromer; although at the doses tested (15 g three times daily for resinsodium and 16.8 g once daily for patiromer, both on non-dialysis days), resinsodium lowered potassemia more than patiromer in hemodialysis patients over four weeks, but tolerance of resinsodium was lower and noncompliance four times higher, raising questions about longer-term compliance (e.g., years).64
Patiromer. An initial dose of 8.4 g/day is recommended. Patiromer begins to reduce the serum potassium several hours after ingestion, with the effect being maintained for 12−24 h.65 Patiromer is generally well tolerated, with the most frequently reported adverse effects being gastrointestinal (constipation, diarrhea, flatulence and abdominal pain) and hypomagnesemia.65,66
In clinical trials, patiromer reduced potassemia in different patient profiles (Table 9).
- none-
Heart failure. The PEARL-HF study67 evaluated the effect on potassemia in patients with chronic heart failure who initiated treatment with spironolactone, and observed a decrease in potassemia in the patiromer group, with a difference with respect to placebo of -0.45 mEq/L (p < 0.001) and a lower incidence of hyperkalemia (7.3% vs. 24.5%, p = 0.01). The decrease in potassemia with respect to placebo was more marked (-0.52 mEq/L, p < 0.05) in patients with CKD (n = 66), that is, in the group with the highest risk of hyperkalemia, in which the incidence of hyperkalemia was 6.7 and 38.5% (p < 0.05) with patiromer and placebo, respectively. The DIAMOND study demonstrated the usefulness of this agent in potassium reduction, adverse events related to hyperkalemia, and a higher proportion of patients treated with optimal doses of antialdosterone drugs in patients with heart failure with reduced ejection fraction and a history or current episode of hyperkalemia.68
- none-
CKD. Patiromer has been studied in three settings: diabetic kidney disease, resistant arterial hypertension, and CKD in general. The AMETHYST-DN study69 evaluated the effect of patiromer in patients with type 2 diabetes, diabetic kidney disease (CKD G3-G4: eGFR between 15 and 60 mL/min/1.73 m2) on treatment with RAS blockers, who had mild hyperkalemia (serum K 5.1–5.9 mEq/L) and who were randomized to patiromer at increasing doses (from 4.2 to 16.8 g twice daily). Potassemia decreased from 0.35 to 0.97 mEq/L, proportional to the initial degree of hyperkalemia and patiromer dose, and the decrease was statistically significant at the first determination (at approximately 48 h) after drug initiation. The proportion of patients achieving the target range of potassemia was greater than 83% in mild hyperkalemia (serum K+ 5.1–5.5 mEq/L) and greater than 77% in moderate hyperkalemia (serum K+ 5.6–5.9 mEq/L).
Phase 2/3 clinical trials with patiromer.
| StudyRef | Patients | Follow-up | Result |
|---|---|---|---|
| Pitt et al.67 (2011).PEARL-HF(phase 2) | N = 120Chronic heart failure with eGFR<60 ml/min/1.73 m2 or history of hyperK+ leading to discontinuation of ß-blockers, RAS inhibitors or aldosterone antagonists | 4 weeks | Mean reduction of serum K+ of 0.22 mEq/L in patients receiving patiromer, versus a mean increase of 0.27 mEq/L in the placebo group.Difference between groups: -0.45 mEq/L (p < 0.001). |
| Bakris et al.69 (2015)AMETHYST-DN (phase 2) | Hypertensive patients with nephropathy secondary to type 2 diabetes mellitus with CKD stages 3−4, receiving treatment with RAS blockers with or without spironolactone.HyperK+ mild - N = 180HyperK+ moderate - N = 66 | 52 weeks | Mean reduction of serum K+: 0.35−0.55 mEq/L in the mild hyperK+ group; 0.87−0.97 mEq/L in the moderate hyperK+ group. More than 75% of the patients with serum K+ in the target range at the end of the study |
| Agarwal et al.70 (2019)AMBER (phase 2) | N = 295Patients aged ≥18 years, with resistant arterial hypertension, with an eGFR between 25−45 ml/min and serum potassium between 4.3 and 5.1 mEq/L. | 12 weeks | Percentage of patients who continued therapy with spironolactone:66% in the placebo group vs. 86% in the patiromer group. Difference vs. placebo: 19.5% (p < 0.0001). |
| Weir et al.71 (2015).OPAL-HK (phase 3) | Patients between 18−80 years of age, with CKD stages 3−4, serum potassium between 5.1 and 6.5 mEq/L and who had been receiving a stable dose of one or more RAS blockers and/or antialdosterone for at least 28 days.Initial phase open - N = 237Randomization phase - N = 107 | 4 weeks8 weeks | Average reduction of serum K+ of 1 mEq/L with respect to the basal value in the initial phase.Randomization phase: increase of 0.72 mEq/L of serum K+ in the placebo group versus no change (0 mEq/L) in the patiromer group (p < 0.001 between groups). |
| Butler et al.68 (2022)DIAMOND (phase 3) | N = 878Heart failure with reduced ejection fraction, RAS and mineralocorticoid receptor blockade, with hyper K+ or history of hyper K related to RAS inhibitor therapy. | 27 (13−43) weeks | Difference between patiromer and placebo in serum K+: −0.10 mmol/l (95% CI −0.13, 0.07); p < 0.001.Risk of hyperK+ > 5.5 mmol/l: HR 0.63 (95% CI 0.45, 0.87); p = 0.006Reduction of aldosterone antagonist dose: HR 0.62 (95% CI 0.45, 0.87); p = 0.006Reduction in total hyperK-related events+: HR 0.66 (95% CI 0.53−0.81); p < 0.001. |
CKD: chronic kidney disease; eGFR: estimated glomerular filtration rate; HyperK+: hyperkalemia; RAS: renin-angiotensin system.
AMBER70 patients with resistant arterial hypertension and CKD G3b-G4 (eGFR 25−45 mL/min), in whom spironolactone therapy was initiated, were randomized to patiromer or placebo. The primary endpoint was the proportion of patients who were still on spironolactone at 12 weeks, which occurred in 98/166 (66%) placebo patients and 126/147 (86%) patiromer patients (between-group difference: 19.5%, p < 0.0001).
OPAL-HK71 was a phase 3 clinical trial that randomized patients with G3-G4 CKD treated with stable doses of RAS blockers and hyperkalemia to patiromer or placebo. An initial, open-label phase treated 237 patients with patiromer, in whom potassemia decreased by a mean of −1.01 mEq/L (p < 0.001) at four weeks. In the second phase, 107 patients who had reached potassium levels in the target range (3.8–5.1 mEq/L) were randomized to continue with patiromer or switch to placebo for another four weeks. At the end of this second phase, the mean increase in potassemia was greater in the placebo group (p < 0.001) and the recurrence of hyperkalemia (60%) was more frequent than in the patiromer group (15%, p < 0.001).
Sodium zirconium cyclosilicate. SZC has also demonstrated its efficacy in the chronic treatment of hyperkalemia in a wide range of patients. The presentations authorized in Spain are 5 and 10 g. It rapidly (from the first hour) reduces serum potassium, with a mean time of 2.2 h to reach normal potassemia, and with 98% of patients reaching normalization of potassemia in the first 48 h.72 The most frequently reported adverse reactions are hypokalemia (4.1%) and edema (5.7%). The latter has been related to the sodium content of the molecule (each 5 g of SZC contains approximately 400 mg of sodium), which is exchanged with potassium, being mainly observed with the 15 g/day presentation, which is not authorized in Spain.72,73
The main clinical trials with SZC are listed in Table 10.
Phase 3 clinical trials with sodium zirconium cyclosilicate (SZC).
| StudyRef | Patients | Follow-up | Result | |
|---|---|---|---|---|
| Kosiborod et al.74 (2014).HARMONIZE | Outpatients (Cardiology, Nephrology or Investigation) (65%)diabetes, 66% CKD, 36% heart failure)Initial phase open - N = 258Randomization phase - N = 237 | Initial phase: 10 g SZC three times/dayRandomization after the initial phase to 3 doses of SZC: 5, 10 and 15 g/day.48 h29 days | [Mean reduction of serum K+ in the initial open phase of 1.1 mEq/L (p < 0.001 vs. baseline level).Mean potassemia in the maintenance phase after randomization:SZC 5 g/day: 4.8 mEq/LSZC 10 g/day: 4.5 mEq/LSZC 15 g/day: 4.4 mEq/LPlacebo: 5.1 mEq/L (p < 0.001 vs. each dose of SZC). | |
| Packham et al.75 (2015)ZS-003 | N = 75474.5% ERC66.7% with RAS blockade, 59.9% Diabetes,39.8% heart failure | Randomization in the initial phase to 4 doses of SZC: 1.25, 2.5, 5 and 10 g 3 times/day and assessment at 48 h.Maintenance: randomization of patients with normal K+ to receive placebo or SZC and assessment at the end of the first year.14 days | [Mean exponential rate of K decrease+ per hour vs. baseline in the initial correction phase:SZC 1.25 g: 0.11%.SZC 2.5 g: 0.16%*.SZC 5 g: 0.21%*.SZC 10 g: 0.30%*.Placebo: 0.09% (*P < 0.001 vs. placebo)Exponential average rate of change of K+ per hour in the maintenance phase:SZC 10 g: +0.14% vs. +1.04% with placebo (P < 0.001).SZC 5 g: +0.09% vs. +0.47% vs. +0.47% with placebo (P = 0.008) | |
| Fishbane et al.76 (2020)DIALIZE | N = 196Patients with stage 5D CKD on hemodialysis treatment ≥ 3 months and pre-dialysis hyperK + | 1 week screening, 8weeks therapy (4 weeks dose titration/ 4 weeksevaluation), 2 weeks follow-up | Percentage responders SZC group: 41.2%; Percentage responders placebo group: 1%. p < 0.001 between groups.Sensitivity analysis results:Percentage of SZC group responders: 42.3%; Percentage of placebo group responders: 2%. p < 0.001 between groups. | |
CKD: chronic kidney disease; hyperK+: hyperkalemia; RAS: renin-angiotensin system.
HARMONIZE74 studied adults with persistent hyperkalemia seen as outpatients in cardiology, nephrology and research services, who received 10 g of SZC three times daily during an initial 48-h open-label phase; 92% of patients normalized potassemia and were then randomized to 5, 10 or 15 g daily of SZC or placebo for four weeks. Mean potassemia at 29 days was significantly lower with the different doses of SZC than with placebo (p < 0.001). The proportion of patients with normal serum potassium levels was higher with SZC (71, 76, and 85% for the 5 g, 10 g, and 15 g daily dose, respectively, vs. 48% in the placebo group), a statistically significant difference for each dose of SZC vs. placebo.
Another phase 3 clinical trial with 754 patients (74.5% with CKD, 66.7% on RAS blockers, 59.9% with diabetes and 39.8% with a history of heart failure) confirmed the significant reduction of potassemia in 48 h with different doses of SZC compared to placebo and the maintenance of the result up to 14 days.75
DIALIZE76 was a phase 3b clinical trial involving 196 hemodialysis patients with persistent hyperkalemia despite adequate dialysis. The primary efficacy endpoint was the proportion of patients with predialysis potassemia between 4.0 and 5.0 mEq/L without rescue therapy, which was achieved in 41% of SZC patients and 1% of those in the placebo group (p < 0.001).
In a long-term phase 3 study, potassemia was normalized in more than 99% of patients within the first two to three days and these normal levels of serum potassium (K+ ≤ 5.1 mmol/L) was maintained in 87% of participants at the end of a 12-month follow-up.72
In addition to limiting the intestinal absorption of potassium, SZC provides benefits against metabolic acidosis, as indicated in the corresponding section.43
Patiromer vs. SZCPatiromer and SZC have better tolerance, efficacy and safety than cationic resins, favoring the use of RAS and MRA inhibitors in pathologies where their benefit has been demonstrated, such as arterial hypertension, CKD and heart failure.77,78
There are no clinical trials that have directly compared patiromer and SZC, and differences in the design and setting of clinical trials with each drug make comparison difficult. A meta-analysis of six studies and 1,756 patients (654 on patiromer and 1,102 on SZC) compared the efficacy and safety of patiromer and SZC in the treatment of hyperkalemia.78 No baseline differences were observed in patient age, eGFR or potassemia, although there was a lower proportion of patients with CKD, heart failure and diabetes, and receiving RAS blocker therapy in the SZC trials. With patiromer, the mean decrease in potassemia at 3 days of treatment was 0.36 mEq/L, and with SZC it was 0.67 mEq/L at 48 h; 7.6% of patiromer patients had constipation and 7.1% hypomagnesemia, while in those receiving SZC, 1.6% had diarrhea and 0.9% had edema.79 In a more recent meta-analysis, patiromer showed lower rates of hyperkalemia with respect to standard treatment, with no differences in terms of adverse effects or discontinuation of treatment due to these effects. In relation to SZC, there were no differences in the occurrence of hyperkalemia or in the overall rate of adverse effects, although there was a higher frequency of edema in the SZC group.80
There is no good evidence on the impact of patiromer and SZC on postprandial hyperkalemia after high potassium intake, despite normalizing basal potassemia levels.81
Both patiromer and SZC have been shown to be effective in solid organ transplant patients.82
Research needsDespite recent advances in the treatment and mitigation of hyperkalemia, there are still unanswered questions whose answers will improve the prognosis of patients at high risk of hyperkalemia (Box 5).
Research needs.
| In which patients with hyperkalemia can an acute parenteral intervention be substituted for an acute oral intervention? |
| Do the new oral potassium binders allow the renal patient to be released from the potassium-poor diet and to follow a more heart-healthy diet? Do they prevent postprandial hyperkalemia? |
| Do patiromer and SZC have differential preferred indications in the treatment of chronic hyperkalemia? |
| Does the use of oral potassium binders slow the progression of CKD? |
| Does the use of oral potassium binders improve long-term cardiorenal prognosis? |
Hyperkalemia is a frequent finding in daily clinical practice and it is associated with worse health outcomes, including increased morbidity and mortality. Therefore, it is essential to make an early and etiological diagnosis of hyperkalemia in order to treat each patient in the most appropriate way. Since hyperkalemia encompasses different pathophysiological and causal mechanisms, it woul benefits from a multidisciplinary approach and treatment. At present, there are multiple therapeutic options available for the treatment of hyperkalemia that have demonstrated their efficacy and safety in various groups of patients, and should be used in conjunction with good clinical judgment. Despite the advances made in recent years in relation to hyperkalemia, there are still gaps of knowledge in this field, which should be addressed and promoted through research to improve the prognosis of the patient with hyperkalemia.
FinancingThere has been no funding for the development of this work.
Conflicts of interestDr. Alberto Ortiz has received grants from Sanofi and consulting or lecture fees or travel support from Advicciene, Alexion, Astellas, AstraZeneca, Amicus, Amgen, Boehringer Ingelheim, Fresenius Medical Care, GSK, Bayer, Sanofi-Genzyme, Menarini, Mundipharma, Kyowa Kirin, Lilly, Freeline, Idorsia, Chiesi, Otsuka, Novo-Nordisk, Sysmex and Vifor Fresenius Medical Care Renal Pharma, is Director of the AstraZeneca-UAM Chair of chronic kidney disease and electrolytes and has been director of the Mundipharma-UAM Chair of diabetic kidney disease. He holds shares in Telara Pharma.
Dr. José Carlos Fernández García has received research grants from Menarini and AstraZeneca, and honoraria for consultancies, lectures and training activities from Boehringer Ingelheim, Sanofi, Eli Lilly, Esteve and Novo-Nordisk.
Dr. Jorge Gómez Cerezo has received consulting and speaking fees from Amgem, AstraZeneca, Sanofi, Bayer, Menarini. JGC is Professor of Medicine at the European University of Madrid.
Dr. Francisco Pita Gutiérrez has received consulting, speaking and training fees from Abbott, Fresenius Kabi, Nestlé Health Science and Nutricia.
Dr. Juan F. Navarro González has received research grants from Abbvie, BioNet Medical, Boehringer Ingelheim, Sanofi, Shire and Vifor Pharma; and honoraria for consultancies, lectures and training activities from Abbvie, Amgen, AstraZeneca, Bayer, Bionet Medical, Boehringer Ingelheim, Eli Lilly, Esteve, Janssen, Menarini, MSD, Mundipharma, Novartis, Novo-Nordisk, Sanofi, Servier, Shire and Vifor Pharma. JFNG is an academic editor of the Journal of Clinical Medicine and associate editor of Frontiers in Medicine, and a member of the Scientific Advisory Board of the European Renal Association.
Author affiliationsAlberto Ortiz. Nephrology and Hypertension Service, Instituto de Investigación Sanitaria Fundación Jiménez Díaz. Department of Medicine, Universidad Autónoma. Madrid.
Carmen del Arco Galán. Emergency Department, Hospital Universitario de la Princesa. La Princesa Health Research Institute. Faculty of Medicine, Universidad Autónoma. Madrid.
José Carlos Fernández-García. Endocrinology and Nutrition Clinical Management Unit, Hospital Regional Universitario de Málaga. Biomedical Research Institute of Malaga (IBIMA). Faculty of Medicine, University of Malaga.
Jorge Gómez Cerezo. Internal Medicine Department, Hospital Universitario Infanta Sofía. European University of Madrid.
Rosa Iban Ochoa. Emergency Department, Hospital Universitario Río Hortega, Valladolid.
Julio Núñez. Cardiology Service, Hospital Clínico Universitario de Valencia. University of Valencia. INCLIVA. CIBER Cardiovascular Diseases, Carlos III Health Institute.
Francisco Pita Gutiérrez. Clinical Nutrition and Dietetics Unit, Endocrinology and Nutrition Service, Complexo Hospitalario Universitario A Coruña.
Juan F. Navarro-González. Research Unit and Nephrology Department, Hospital Universitario Nuestra Señora de Candelaria, Santa Cruz de Tenerife. RICORS2040, Instituto de Salud Carlos III, Madrid.
Isabel Comerma, Carmen Mon, Enrique Morales and Juan Payá provided comments during the public review phase of the document, which were partially adopted.