Autosomal dominant polycystic kidney disease (ADPKD) is the most frequent cause of genetic renal disease and accounts for 6–10% of patients on kidney replacement therapy (KRT).
Very few prospective, randomized trials or clinical studies address the diagnosis and management of this relatively frequent disorder. No clinical guidelines are available to date. This is a revised consensus statement from the previous 2014 version, presenting the recommendations of the Spanish Working Group on Inherited Kidney Diseases, which were agreed to following a literature search and discussions. Levels of evidence mostly are C and D according to the Centre for Evidence-Based Medicine (University of Oxford). The recommendations relate to, among other topics, the use of imaging and genetic diagnosis, management of hypertension, pain, cyst infections and bleeding, extra-renal involvement including polycystic liver disease and cranial aneurysms, management of chronic kidney disease (CKD) and KRT and management of children with ADPKD. Recommendations on specific ADPKD therapies are provided as well as the recommendation to assess rapid progression.
La poliquístosis renal autosómica dominante (PQRAD) es la causa más frecuente de nefropatía genética y representa entre 6 a 10% de los pacientes en terapia de reemplazo renal (TRR).
Muy pocos ensayos prospectivos, aleatorizados o estudios clínicos abordan el diagnóstico y el tratamiento de este trastorno relativamente frecuente. No hay guías clínicas disponibles hasta la fecha. Esta es un docuemento de consenso revisada de la versión anterior de 2014, que presenta las recomendaciones del Grupo de Trabajo Español de Enfermedades Renales Hereditarias, acordadas tras la búsqueda bibliográfica y discusiones. Los niveles de evidencia en su mayoría son C y D según el Centro de Medicina Basada en Evidencia (Universidad de Oxford). Las recomendaciones se relacionan, entre otros temas, con el uso de diagnóstico por imágenes y genético, el manejo de la hipertensión, el dolor, las infecciones y el sangrado quístico, la afectación extrarrenal, incluida la enfermedad poliquística hepática y los aneurismas craneales, el manejo de la enfermedad renal crónica (ERC) y el TRR asi como el seguimiento de niños con PQRAD. Se proporcionan recomendaciones sobre terapias específicas para la PQRAD, así como la recomendación para evaluar la rápida progresión.
Autosomal dominant polycystic kidney disease (ADPKD) is the most common hereditary kidney disease.The estimated prevalence is highly controversial and ranges between 1 per 500 and 1 per 2000 people1–4.
Patients with ADPKD make up between 6% and 10%, approximately, of the population on dialysis or with a kidney transplant, being, therefore, a disease with a great social impact5,6. It is characterized by the progressive development of renal cysts that usually lead to end-stage CKD (ESRD), generally in adulthood. It is also associated with systemic manifestations, such as high blood pressure (HBP), intracranial aneurysms (ICA), polycystic liver disease, valve abnormalities and cysts in other organs. During the last 3 decades there have been great advances in the knowledge of the disease. In the mid-1990s, the genes that cause ADPKD were identified: PKD1 and PKD7,8. Other genes that cause autosomal dominant cystic nephropathy have been identified that clinically differ from ADPKD (GANAB, DNAJB11)9,10. PKD1 and PKD2 encode polycystins 1 and 2, which are located in the primary cilium11. Molecules that are overexpressed or deficient in polycystic cells are now potential therapeutic targets, so that there are currently several drugs under study as treatment for the disease. The therapeutic use of tolvaptan was approved In 2014, in Japan and in 2015 in Canada and Europe. It was approved for the treatment of patients over 18 years of age with ADPKD stages 1–3 and with signs of rapid progression. In June 2018, the European Medication Agency (EMA) extended the indications of tolvaptan to patients with ADPKD stages 1–4 at the initiation of treatment and withevidence of rapid progression (https://www.ema.europa.eu/en/documents/procedural-steps-after/jinarc-epar-procedural-steps-taken-scientific-information-after-authorisation_en.pdf) and in the same year it was approved in the United States for adults with rapidly progressing disease without defining the stage of CKD at the start of treatment12,13. There are a limited number of prospective randomized controlled trials, as well as clinical studies that incorporate an experimental design for the diagnosis and management of ADPKD. Such studies are difficult to perform due to the relatively low number of patients in each center, as well as the heterogeneity of the clinical presentation of the disease. The consensus recommendations in these clinical guidelines are based on a search of the literature and, to a large extent, on the experience and opinions of the authors. The Cochrane Library, MEDLINE, and the Systematic Reviews database (up to July 1, 2019) have been used to collect the ublications. We using the search terms ‘ADPKD’ or ‘polycystic kidney’ in combination with the terms ‘diagnosis’ or ‘imaging’ or ‘gene’ or ‘HTN’ or ‘CKD’ or ‘chronic kidney disease’ or ‘chronic renal failure’.” or “ESRD” or “end-stage kidney disease” or “dialysis” or “transplant” or “infection” or “pain” or “liver” or “aneurysm” or “cancer” or “pregnancy” or “children” or “tolvaptan”. To a large extent, we selected publications from the last 10 years, but relevant older publications were not excluded. The recent KDIGO (Kidney Disease Improved Global Outcomes) in ADPKD14 have been especially taken into account. The reference lists of the identified articles were also reviewed and those deemed relevant were selected. Review articles are cited to provide readers with more detail than that provided in these guidelines. The authors have tried to make this guide concise and very practical and, standardize, as much as possible, the care of patients with ADPKD.
The authors are members of the Hereditary Kidney Diseases Working Group of the Spanish Society of Nephrology. They sign the guides in alphabetical order, except the last author. The authors reached a consensus on the recommendations and considered that the benefits outweighed the potential risks. The document has been submitted to public review by the members of the Spanish Society of Nephrology. The levels of evidence are mostly low: levels C and D according to the Center for Evidence-Based Medicine (Oxford University) (http://www.cebm.net/?o=1025). These guidelines address the following aspects of the disease: diagnosis, hypertension, pain, assessment of kidney disease progression, end-stage kidney disease, polycystic liver disease, ICA, other extrarenal features, specific treatment of the disease, and ADPKD in children. The initial version of these guidelines was published in 2014 and updated in 201715.
DiagnosisSince ADPKD is an autosomal dominant disease, children of affected parents have a 50% chance of developing the disease. Due to its high penetrance, it is highly unlikely to skip a generation. Currently, ultrasound is used to detect and diagnose the disease in people with an affected family member. The diagnosis of sporadic cases, which represent ∼10% of patients, is based on the clinical characteristics of the disease, but sometimes a genetic study is necessary, especially in the early stages of the disease. ADPKD must be distinguished from other causes of renal cysts such as simple cysts, autosomal recessive polycystic kidney disease, nephropathy due to HNF1B mutations, and other cystic kidney diseases (Table 1). In an adult, the presence of enlarged cystic kidneys, a decreased estimated glomerular filtration rate (eGFR), hypertension, and liver cysts is highly indicative of ADPKD. But often, not all of these clinical features are present and diagnosis becomes more complicated. In these cases, the genetic study is very useful.
Differential diagnosis of ADPKD.
| Illness | Differentiating signs or symptoms | Differentiating tests |
|---|---|---|
| Acquired cystic disease | • Medical history | • Kidney size is usually small, unlike the cystic nephromegaly of ADPKD; rarely there may have some nephromegaly |
| • Pre-existing CKD | • Frequent hemorrhagic cysts | |
| Simple cysts | • Common in adults. Its incidence increases with age. Uncommon in those under 40 years of age | • Diagnosis based on ultrasound: absence of internal echoes, well-defined wall, acoustic enhancement, spherical or ovoid shape |
| No family history and does not meet Pei’s criteria | • It is used the Bosniak classification | |
| • They are rarely complicated | ||
| Tuberous sclerosis | • Facial angiofibromas, hypomelanic spots, nail fibromas, rough plaques, cortical tubers, subependymal nodules, giant astrocytomas, pulmonary lymphangioleiomyomatosis, cardiac rhabdomyomas, renal angiomyolipomas | • Mutational analysis of TSC1 and TSC2 genes |
| • More frequent angiomyolipomas than cysts. More frequent in TSC2 than in TSC1 | ||
| • TSC2/PKD1 contiguous gene syndrome : early-onset ADPKD and deletion tuberous sclerosis involving both genes | ||
| V on Hippel-Lindau syndrome | • Renal carcinoma, retinal or central nervous system hemangioblastoma, pheochromocytomas, pancreatic cysts, and epididymal cystadenomas | VHL gene mutational analysis |
| Disease due to mutations in the HNF1B gene | • Type 2 DM, renal cysts, renal hypoplasia, renal agenesia, hyperechoic kidneys, genital abnormalities, hypomagnesemia | • HNF1-B gene mutational analysis |
| Autosomal dominant tubulointerstitial nephropathy due to mutations in the UMOD/MUC1 genes | • Tubulointerstitial nephropathy, frequent gout or hyperuricemia, sometimes cysts at the corticomedullary junction | • MRI sometimes shows cysts at the corticomedullary junction |
| • Mutational analysis of the UMOD gene and the MUC1 gene | ||
| Autosomal dominant tubulointerstitial nephropathy due to mutations in the REN gene | • CKD, hypotension, cysts, hyperuricemia, hyperkalemia | • Mutational analysis of the REN gene |
| Orofaciodigital syndrome type 1 | • Only affects women. Lethal in males | • Mutational analysis of the OFD1 gene |
| Radiological pattern similar to ADPKD, but less increase inrenal volume in 70% of adult female patients. | ||
| Renal spongiosis or precaliciliary renal ectasia or Cacchi-Ricci disease | • Absence of family history or autosomal dominant | • IVU |
| • Radiated image for urography | ||
| • Frequent stones | ||
| • Dilation of collecting ducts at the level of the pyramids | ||
| Autosomal recessive polycystic kidney disease | • Neonatal-childhood diagnosis usually | • PKHD1 gene mutational analysis |
| Always liver fibrosis more or less symptomatic. | ||
| In adults renal insufficiency with cystic kidneys of normal size or minimally enlarged. | ||
| HANAC syndrome | • Cysts, hematuria, CKD, muscle cramps, intracranial aneurysms, cataracts, retinal tortuosities. | • Mutational analysis in the COL4A1 gene |
| Autosomal dominant polycystic liver disease: | ADPKD ESRD PQH | • Mutational analysis of PRKCSH, SEC63, LRP5 ADPLD-GANAB, ALG8 and SEC61B |
| • ADPLD-PRKCSH | Absent non-moderate or mild to severe | |
| • ADPLD-SEC63 | Absent non-moderate or mild to severe | |
| • ADPLD-LRP5 | Absent non-moderate or mild to severe | |
| • ADPLD-GANAB | Absent non-moderate or mild to severe | |
| • ADPLD-ALG8 | ||
| • ADPLD-SEC61B |
MRI: magnetic resonance; IVU: intravenous urography.
Early diagnosis in adults may improve cardiovascular (CV) risk factors and allows the consideration of a specific treatments to modify disease progression.
Recommendations- 1
Patients diagnosed with ADPKD should be advised to inform their first-degree relatives about the diagnosis, and the relatives should be offered ADPKD screening (D).
- 2
Genetic counseling (GC) should always be offered (C).
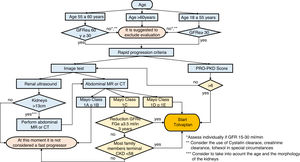
Ultrasound is the most widely used imaging technique for the diagnosis and follow-up of ADPKD. This technique is widely available, inexpensive, and does not require radiation or contrast. The new generation of ultrasound scanners provides a resolution of up to 2–3mm, although this depends on the body characteristics of the patient and the experience of the sonographer. Ultrasonography is also useful to explore abdominal extrarenal involvement of ADPKD, such as liver or pancreatic cysts, which support the diagnosis of ADPKD. However, it is not a good method of measuring total renal volume (TRV), especially if the renal diameter is greater than 17cm.
Diagnostic criteria by ultrasound have been defined for relatives of patients with mutations in the PKD1 gene16 or unknown genotype17 (Table 2). The sensitivity of classical ultrasound for PKD1 patients is significantly higher than in PKD2 patients.
Diagnostic criteria for ADPKD by ultrasound.
| Ravine criteria (1994) for patients at risk of PKD1 mutation: |
| • At least 2 cysts in the kidneys or 1 cyst in each kidney in patients younger than 30 years |
| • At least 2 cysts in each kidney in patients aged 30-59 years |
| • At least 4 cysts in each kidney in patients 60 years of age or older |
| Pei cirteria (2009) for ADPKD patients with unknown genotype and positive family history (=modified Ravine criteria): |
| • Three or more renal cysts (unilateral or bilateral) in patients aged 15 to 39 years |
| • Two or more cysts in each kidney in patients aged 40 to 59 years |
The presence of less than 2 renal cysts offers a negative predictive value of 100% and can be considered sufficient to rule out the disease in individuals at risk older than 40 years.
Computed tomography (CT) is more sensitive than classical ultrasound and can detect cysts as small as 1–2mm18, as well as stones. Aditionally, it is better than ultrasound in identifying kidney tumors. However, CT exposes patients to radiation and is more expensive, so while it can determine TRV, it is not routinely used for follow-up.
Magnetic resonance imaging (MRI) is more sensitive than ultrasound or CT. It may be even more helpful in distinguishing renal cell carcinoma from simple cysts. It turns out to be the best imaging technique to define TRV, especially repeatedly, as it does not expose the patient to radiation. However, in many centers it is not easily accessible. There are different approaches to determine the TRV, but currently it is considered that the approximation using the ellipsoid formula is the most cost-effective, although planimetry and semi-automatic studies are gaining ground19–22. Recently, it has been suggested diagnostic criteria for ADPKD using MRI23 (Table 3).
Diagnostic criteria for ADPKD by magnetic resonance imaging.
| Subjects between 16 and 40 years of age at risk of presenting ADPKD: |
| • >10 cysts between the two kidneys: ADPKD |
| • <10 cysts between the 2 kidneys: no ADPKD |
| Subjects between 16 and 40 years old at risk of presenting ADPKD and who wish to be kidney donors |
| • <5 cysts between the two kidneys: acceptable as a donor |
- 1
Ultrasound is the recommended diagnostic screening tool for relatives of an index case. The criteria defined inTable 2(C) should be used.
- 2
CT or MRI should be used in doubtful cases or in those with suspicion of another associated renal pathology such as stones or tumors (D) or to define which patients are rapid progressors by measuring the TRV.
- 3
The use of CT or MRI will depend on the availability of the technique. They would be the best choice:
- a
Use CT when stones should be evaluated (D).
- b
Use MRI if regular VRT monitoring is planned, such as in the context of clinical trials (D).
- a
- 4
In the event of inconclusive results from the imaging tests, a genetic study is recommended (D).
At present, the genetic diagnosis of ADPKD is affordable, but still has a non-negligible cost, which is why it is indicated in certain circumstances.
The indications for the genetic study of ADPKD are:
- 1
Young subjects who need to confirm/rule out the diagnosis of ADPKD, such as relatives of patients with ADPKD who are candidates for living donors with inconclusive ultrasound.
- 2
Patients with no family history of ADPKD, due to phenotypic overlap with other cystic kidney diseases (Table 1).
- 3
Patients with equivocal or atypical imaging (eg, markedly asymmetric polycystic kidney disease, renal failure without significant nephromegaly); highly discordant disease severity among members of the same family; ADPKD very mild or very early onset or very severe or with syndromic features.
- 4
Patients who want GA, especially couples who want preimplantation genetic diagnosis (PGD).
- 5
Patients with hypertension or urological symptoms of the disease before the age of 35, in whom the presence of a mutation in PKD1 would allow the application of the PROPKD score for the risk of rapid progression and, therefore, indicate a specific treatment.
Recently, 2 new genes have been described that infrequently cause atypical forms of polycystic kidney disease: GANAB and DNAJB119,10. The disease caused by mutations in these genes differs from classic ADPKD and it has been questioned whether they should be called ADPKD24. Mutations in the GANAB gene have been described in very few families and represent approximately 0.3% of all cases of polycystic kidney disease. They cause a fairly mild renal phenotype, without renal failure, and a polycystic liver disease phenotype of variable severity ranging from no liver cysts to severe polycystic liver disease. The DNAJB11 gene has been described only in 5 families as the cause of a combination of polycystic kidney disease and autosomal dominant tubulointerstitial kidney disease (ADTKD) with normal-sized cystic kidneys and progressive interstitial fibrosis with late-onset kidney failure.
Routine genetic diagnosis to identify the gene causing the disease (PKD1 78% of cases; PKD2 15%; GANAB 0.3%; DNAJB11 0.1%) is currently questionable since there is considerable clinical variability associated with each gene and the type of mutation and, in general, the result does not usually significantly modify the therapeutic approach. However, it should be noted that an important genotype-phenotype correlation has been described with a possible influence on the decision to start treatment25: truncating PKD1 pathogenic variants (those that generate a protein with a smaller size than the normal protein or there is no of protein) have a worse prognosis than non-truncating PKD1 pathogenic variants (those that generate a protein with an amino acid change or an insertion/deletion of a number of amino acids less than 5) and this information is part of some risk scores for rapid progression that allows a specific treatment to be indicated (see the Progression section)26.
Recommendations- 1
Routine genetic diagnosis of ADPKD is not recommended if the clinical and imaging diagnosis is clear (no grade of recommendation).
- 2
The specific situations in which the genetic diagnosis of ADPKD is indicated are: potential living donor from a family with ADPKD, young patients without a family history of ADPKD or with an uncertain clinical diagnosis, very early onset of the disease, PGD, urological symptoms or hypertension before 35 years of age to define the risk of rapid progression and indication of specific treatment (Table 4) (D).
Table 4.Indication of genetic diagnosis of ADPKD.
a) Individual characteristics Potential living donor: each case must be assessed individually, taking into account age, severity of the disease in the family and imaging tests Patients with no family history of ADPKD. Especially indicated: - When radiological findings are atypical (eg, marked renal asymmetry, multiple small cysts, renal failure in the presence of cystic kidneys of normal size) - In patients with very mild involvement. - In patients with atypical extrarenal manifestations of ADPKD. - When a relative prognostic information is required, since truncating PKD1 mutations are associated with a worse prognosis than non-truncating PKD1 mutations and PKD2 mutations are associated with a better prognosis than PKD1 mutations. Patients with a very early onset of the disease - In families with a typical presentation of ADPKD, but with a relative with a very early presentation, genetic testing may identify a hypomorphic allele in addition to the allele with the pathogenic mutation or a hypomorphic allele in both copies of PKD1 - In patients with no family history of ADPKD and in whom no mutations have been identified in the PKHD1 gene (causing autosomal recessive polycystic kidney disease) or with radiological features of ADPKD Patients with or without a family history who desire future preimplantation or prenatal genetic diagnosis b) Family characteristics Families with multiple relatives with renal cysts with an atypical radiological pattern of ADPKD, candidates for a differential diagnosis of other cystic renal diseases
Currently, the most widely used methodology for the genetic diagnosis of ADPKD is the sequencing of the causing genes by massive sequencing (also called next-generation sequencing) or classic Sanger sequencing.
Mass sequencing allows simultaneous sequencing of PKD1, PKD2, GANAB and DNAJB11, along with a large number of other genes also associated with cystic kidney disease; it reduces the time and cost of mutational analysis and provides additional information in patients with atypical phenotypes27–30. More than 100 genes associated with renal cysts are currently known. Patients with an earlier and more severe presentation of the disease than their relatives, in addition to the family mutation, may also have an altered copy of the PKD1 gene with a hypomorphic allele (sequence variant that generates a partially functioning protein)31,32 or with a mutation in another ADPKD-causing gene such as HNF1B or PKHD133,34.
Classical Sanger sequencing involves sequential (not simultaneous) analysis of the 46 exons and intronic flanking regions of PKD1 and the 15 of PKD2. Generally, is initiated the analysis of PKD1, since it is the most frequently mutated in ADPKD35. However, if a relative has reached ESRD after the age of 70, the analysis begins with PKD2 sequencing.
If sequencing does not identify any clearly pathogenic (truncating) variant or a variant with an amino acid change widely described in the literature as pathogenic, the MLPA (Multiplex Ligation-dependent Probe Amplification) technique should also be performed, which detects large deletions and duplications in approximately 4% of cases36.
The main limitation of mutational analysis is that the sensitivity of the technique is always less than 100%, so not identifying a mutation does not allow either ruling out or confirming the suspected diagnosis. However, studies in large cohorts have reported a high sensitivity of between 88% and 94%26.
The difficulties in mutational analysis of the genes that cause ADPKD lie mainly in: 1) the high allelic heterogeneity of these genes, such that the same pathogenic variant is not found in more than 2% of families; 2) the complexity of PKD1, due to the existence of 6 pseudogenes with a 98% identical sequence in exons 1–33 of this gene; 3) the difficulty of classifying non-truncating variants into pathogenic, probably pathogenic, of uncertain clinical significance (VUS), probably benign, benign or hypomorphic, and 4) the existence of mosaicism. For all these reasons, it is important that the genetic diagnosis is carried out by laboratories with the appropriate experience.
Genetic diagnosis based on family linkage analysis is currently obsolete, since it requires the participation of at least 3 family members diagnosed with ADPKD with absolute certainty. Linkage analysis would only be recommended in familial cases with 100% positive symptoms of ADPKD, in which sequencing of the genes that cause ADPKD has not identified any pathogenic variant. It cannot be used in patients who do not have a family history or in cases with doubtful symptoms of ADPKD. Furthermore, it is not applicable, or may lead to false results, if there are de novo mutations, hypomorphic alleles31,32, recombinations, or mosaicism37,38.
Recommendations- 1
Mutational analysis of PKD1, PKD2, GANAB and DNAJB11 is currently the genetic diagnostic method of choice in ADPKD (D).
- 2
Mass sequencing of PKD1, PKD2, GANAB, and DNAJB11 is highly recommended, along with a broad panel of other genes also associated with cystic kidney disease in patients with atypical phenotypes (D).
The advice or GC, according to the Biomedical Research Law (LIB 14/2007)39, is “the procedure intended to inform a person about the possible consequences for him or his offspring of the results of a genetic analysis or screening and its advantages and risks, and, where appropriate, to provide an advise in relation to the possible alternatives derived from the analysis”. Ideally, the GC should be provided by multidisciplinary team in which all the agents and specialists involved interact (including clinical and molecular geneticists, bioinformaticians and genetic counselors).
One of the objectives of GC is to prevent the patient or family regarding reproductive decision-making. During the GC process, the patient should be informed about the options available to him, but in no case should be advised about the decision to be made.
In terms of reproductive decisions, the possibilities for a patient with ADPKD in our country are the following: 1) assume a 50% chance of having an affected child; 2) selection of embryos by preimplantation genetic testing (PGT); 3) prenatal diagnosis; 4) gamete donation; 5) adoption, and 6) give up having offspring. Under no circumstances we may give directions to reach a decision and never judge the patient for the decision made.
The PGT is an assisted reproduction technique that includes a genetic diagnosis of the embryos and the selection of disease-free embryos to be transfer to the mother’s uterus. The PGT for ADPKD is approved by the National Commission for Assisted Reproduction. One of the advantages of PGT over prenatal diagnosis is that it avoids the termination of pregnancy. However, it has drawbacks such as the need to identify the causal sequence variant (not feasible in 10% of cases), an in vitro fertilization process (hormonal treatment not recommended in women with significant polycystic liver disease), the pregnancy rate it is approximately 40%, it has a high economic cost, it can be ethically questionable (especially in mild cases) and it is associated with a significant physical and psychological impact, especially for women.
RecommendationsEvery patient with ADPKD should have access to GC (A).
The patient of reproductive age should be informed of the options available to avoid the transmission of the disease (A).
The patient should not be directed to make a specific decision; however, the professional should inform so that the couple can make an autonomous decision with exhaustive knowledge of the possibilities (A).
The TGP is available for patients with ADPKD in Spain. When offering the PGT option to patients, its advantages, disadvantages, and average rate of pregnancy (A) should be explained.
Psychological support is advisable for patients with ADPKD at critical moments in their lives, such as: the process of diagnosis, reproductive decision-making, management of pain and the need for RRT (B).
Management of arterial hypertension and cardiovascular riskIntroductionThe HTN is a very common manifestation in patients with ADPKD. A 60% of patients develop HTN before presenting alterations in renal function40. Ambulatory blood pressure (BP) measurement techniques such as self-measurement of BP (SMBP) or ambulatory BP monitoring (ABPM) facilitate the early diagnosis of HTN and masked HTN, which prevalence is higher than in the general population of HTN patients41. The onset of hypertension is earlier in patients with mutations in PKD1 than in PKD2 and in polycystic patients with hypertensive parents, affected or not by the disease42. Hypertension is associated with a faster progression to ESRD and contributes to increased CV morbidity and mortality.
PathogenesisSeveral mechanisms have been implicated in the pathogenesis of hypertension in ADPKD, but the main one seems to be the activation of the renin-angiotensin system (RAS) secondary to intrarenal ischemia caused by compression of the intrarenal vasculature by expanding cysts43. Although the evidence of a systemic activation of the renin-angiotensin-aldosterone system (RAAS) is contradictory, hyperactivation of the intrarenal RAAS could itself promote hypertension44. Endothelial dysfunction from early stages of the disease could also contribute to the genesis of hypertension45. Dysfunction of the polycystin 1-polycystin 2 complex of the primary cilia of endothelial cells worsens the response to shear stress, reducing the release of nitric oxide, which contributes to hypertension. Other mechanisms involved in the development of HTN in polycystic disease are high concentrations of erythropoietin, alterations in tubular handling of sodium, increased tone of the sympathetic nervous system and increased levels of vasopressin42,46.
TreatmentChanges in lifestyle are a fundamental part in the treatment of essential hypertension. Although the efficacy of these recommendations has not been specificaly studied in hypertensive patients with ADPKD, it is advisable to maintain an adequate weight, perform aerobic physical exercise on a regular basis, quit smoking and limit salt intake to 5–6g/day.
Given that the main etiological mechanism in the genesis of hypertension seems to be the activation of the RAAS, ACE inhibitors (ACEIs) and angiotensin II receptor antagonists (ARBs) should be the first-line antihypertensive drugs. The combined use of ACE inhibitors and ARBs was tested in the HALT study47,48 and did not show additional benefits over the use of one of them, neither in ADPKD progression nor in the CV profile, so their joint use is not recommended. Calcium channel blockers (due to their theoretical deleterious effect in worsening the disease) and diuretics (because they activate the RAAS) should be reserved for cases of resistant HTN and for those patients with renal failure and fluid overload and beta-blockers would probably be second-line antihypertensive drugs. Despite these theoretical considerations about diuretics, in the PKD-HALT trials, the second line of treatment after RAAS blockade was a diuretic, also in line with the recent European guidelines on HTN in CKD49 and the third, metoprolol.
Blood pressure goalThe best evidence available regarding the target BP to be achieved in patients with ADPKD comes from the results of the HALT-PKD studies47,48. In general, and as in the rest of the population with kidney disease, a goal of BP<140/90mmHg is recommended (ideally, and in case of good tolerance to treatment, aiming for a goal around 130/80mmHg)49. However, in young patients (<50 years) with normal renal function, the HALT A study has shown that very strict BP control with an ambulatory BP target of around 95–110/60–75mmHg has advantages versus standard control (<130/80mmHg) in terms of disease progression and improvement of the CV profile. Therefore, in this group of patients, it seems reasonable to seek this stricter BP target, taking into account individual tolerance.
Global cardiovascular riskPart of the increased CV risk presented by patients with ADPKD is linked to the presence of hypertension as a risk factor, but also to the appearance and early progression of subclinical organ damage. Even normotensive polycystic patients present greater subclinical organ damage than healthy normotensive controls50. Early detection and correct treatment of hypertension and other CV risk factors should allow to act on reducing the progression of CV disease and prevent subclinical organ damage and the appearance of CV events.
For the evaluation and treatment of the rest of the CV risk factors, there is no specific evidence for ADPKD, so will be applicable strategies of the renal population in general (http://kdigo.org/home/guidelines) and that of the general hypertensive population.
Recommendations- 1
Lifestyle changes should be implemented: maintain a healthy weight, engage in regular aerobic exercise, and limit salt intake to a maximum of 5–6g/day (C).
- 2
Performing SMBP or ABPM is recommended to achieve an early diagnosis of AHT (D).
- 3
A very strict ambulatory BP target (BP<110/75mmHg) in young patients (<50 years) with normal renal function would be feasible. In the rest of the patients, the BP goal should be similar to that of the other patients with CKD (B).
- 4
Antihypertensive pharmacological treatment should include an RAAS inhibitor as the first option; this is based on its theoretical advantages (C).
- 5
CV risk should be assessed and all modifiable CV risk factors should be treated according to the CKD guidelines (no grade of recommendation).
Women with ADPKD who have kidney failure or hypertension may have an increased risk of developing preeclampsia and fetal loss during pregnancy due to their CKD, but it has not been specifically studied whether having ADPKD is an additional risk51. Pregnancy is not contraindicated in normotensive women with normal renal function. Special attention should be paid to those women who receive treatment with estrogens or progesterone, because polycystic liver could be aggravated14.
Urinary tract infections should also be monitored due to an increased predisposition during pregnancy80.
Recommendations- 1
Pregnancy is not recommended in women with ADPKD with CKD stages 3–5 and excluding transplant patients (D).
- 2
Pregnant ADPKD with Hypertension should be controlled as a high-risk pregnancy (C).
- 3
Pregnant women with ADPKD who are normotensive and have normal renal function do not require special follow-up, although special attention should be paid to BP control (no degree of evidence).
- 4
It is recommended to suspend ACEI/ARA-II due to the risks of teratogenicity (C). Likewise, it is recommended to suspend tolvaptan during pregnancy (B) and use contraception during its use (B).
- 5
In vitro fertilization process is not recommended, due to its inherent hormonal treatment, in women with significant polycystic liver disease. (no degree of evidence).
In ADPKD the renal carcinoma is not more frequent than in other populations with CKD, but it may be more difficult to make the diagnosis52,53.
Recommendations- 1
If macroscopic hematuria lasts more than a week or if the initial episode occurs after the age of 50 years, an imaging test should be performed to rule out renal carcinoma (D).
- 2
A solid mass on ultrasound, speckled calcifications on CT, contrast enhancement, the presence of a tumor thrombus, or regional adenopathy on CT or MRI should raise suspicion of renal carcinoma (C).
The main causes of acute pain are pyelonephritis, cystic infection, cystic hemorrhage, and urolithiasis54,55. Both kidney and liver cysts may be symptomatic. Hemorrhage or rupture of cysts usually presents as acute pain that may be accompanied by macroscopic hematuria and/or anemia. Cystic infection presents with fever and lower back or abdominal pain. Imaging tests may help in the differential diagnosis of the causes of lower back or abdominal pain and in locating the infected cyst. The indications and limitations of the different imaging techniques in patients with ADPKD with pain, fever or bleeding are summarized in Table 556–58. When cystic infection is present, urine and blood culture may be negative, which make a confirmatory diagnosis difficult; in which case, it would require identification of the cyst, puncture, and a positive culture of the content. Given this difficulty, an operative definition of probable cyst infection has been proposed (recommendation 2B) and some authors add the absence of evidence of recent intracystic bleeding on CT without contrast56,58–61. However, the coexistence of infection and hemorrhage is possible. A recent international Delphi consensus endorsed this approach, suggesting that increased C-reactive protein (CRP) can be replaced by a leukocytosis greater than 11,000/μl and including a list of 18 items that increase diagnostic suspicion if routine tests failto identify another cause to explain the signs and symptoms. The diagnosis would be confirmed after image localization of the infected cyst62.
Radiological tests in patients with ADPKD and pain, fever or bleeding.
| Test | Advantage | Additional benefits | Disadvantages | Indication |
|---|---|---|---|---|
| Plain abdominal X-ray | Affordable, accessible | Radiation, does not evaluate cysts | Initial evaluation of abdominal pain | |
| Abdominal/renal ultrasound | Can identify obstruction of the urinary tract, lithiasis and complicated cyst | Diagnosis of ADPKD, it informs on kidney size | Does not differentiate between cyst with infection or hemorrhage | Initial assessment of abdominal pain/fever |
| CT | Sensitive to distinguish lithiasis, images suggestive of recent intracystic hemorrhage can be observed, and sometimes suggestive of infection (gas, intracystic level, increased density of nearby fat) | Estimation of renal volume; estimation of interstitial fibrosis. | Radiation, often identifies multiple images of complicated cysts, does not differentiate well between infection or old cystic hemorrhage, often contrast cannot be used due to renal insufficiency, contrast may elucidate normal pericyst parenchyma | Initial assessment of abdominal pain/fever |
| Lithiasis a | ||||
| MR | T1 and T2 sequences similar to CT. DWI MRI shows changes in infected cysts | Less available, no difference between infection or hemorrhage on T1/T2, often contrast cannot be used due to renal insufficiency, contrast can elucidate normal pericyst parenchyma | Evaluation of fever if CT does not resolve | |
| 18F-FDG PET/CT | Test of choice to locate with precision an infected kidney or liver cyst, it can locate other foci of infection | Radiation, expensive, little availability, no defined criteria for diagnosis and follow-up of infected cysts, also detects tumors and hematomas, possible interference of renal failure in the elimination of the marker | Decision-making in patients with poor evolution when traditional imaging has failed, especially if persistent fever | |
| Scintigraphy with leukocytes labeled with radioactive gallium or indium | Location the inflammation | Little availability, preparation takes 48h, requires external manipulation of leukocytes, poor precision, only positive in 50% of cases | Consider if PET/CT is not available when traditional imaging has failed | |
| Arteriography | Diagnosis and possible treatment of severe active cystic hemorrhage | Radiation, invasive, possibility of l contrast nephrotoxicity | Severe bleeding |
DWI: diffusion-weighted imaging; MRI: magnetic resonance; CT: computed tomography; 18 F-FDG-PET/CT: positron emission tomography with fluorodeoxyglucose labeled with fluorine-18.
Treatment of cystic hemorrhage is generally symptomatic. Improvement has been reported with tranexamic acid, an antifibrinolytic agent, but there are no controlled studies available63.
Empirical treatment of cystic infection should cover the most frequent causative germs, enteric gram-negative bacteria, with antibiotics that penetrate well into cysts, such as quinolones64. Cystic penetration of meropenem is low, with intracystic levels 10 times lower than in plasma65. Increased levels of circulating CA19.9 have been described in patients with liver cyst infection66, but also in patients with ADPKD in general (mean values 3 times higher than in controls and higher in patients with large liver and higher values of gamma glutamyl transpeptidase [GGT])67. A 20–36% of ADPKD patients have urolithiasis, which can also cause acute pain. Uric acid stones are more common than calcium oxalate stones68. Predisposing factors include hypocitraturia, hyperoxaluria, hypercalciuria, hypomagnesuria, possible distal acidification defects, and, above all, urinary stasis due to compression of the collecting system by cysts. Potassium citrate is the treatment of choice for uric nephrolithiasis, calcium oxalate nephrolithiasis due to hypocitraturia, and distal acidification defects. The KHA-CARI guidelines recommend using CT without contrast to confirm the diagnosis of nephrolithiasis (1B) and, if identified, perform a metabolic study (1C), and correct the defects if possible (2D)69. Dual-energy CT differentiates uric acid stones from those containing calcium14.
Several clinical guidelines and documents have recently addressed the treatment of chronic pain14,70. The main causes of it are the increase in the size of the kidneys or the liver. Various invasive procedures have been reported in the literature for pain control if medical treatment fails, generally in case reports or small case series (reviewed in Chapman et al.14). These include celiac plexus block, radiofrequency ablation, spinal cord stimulation, and various forms of denervation (thoracoscopic sympathoplanknicectomy, laparoscopic renal denervation, and transluminal catheter denervation) and even nephrectomy, especially if the patient is already on renal replacement therapy. The KHA-CARI guidelines recommend evaluating chronic pain and involving the patient in the pain management, initially emphasizing non-pharmacological treatment (both 1D) and suggest surgical decortication of cysts if pain is severe and sustained (2C)70. A recently described denervation protocol improved chronic pain in 81% of a total of 44 patients. As a diagnostic maneuver, a temporary celiac plexus block is performed; If pain recurs after initial improvement, radiofrequency ablation of the celiac plexus is performed. If the pain does not improve, renal denervation is performed with a catheter71. In a preliminary study, renal catheter denervation reduced chronic pain in a mixed group of patients (n=11) that included patients with ADPKD72. A recent concept is the prevention of pain; the phase 3 randomized controlled trial TEMPO 3:4 study observed a 36% decrease in the incidence of pain episodes (secondary endpoint) in patients treated with tolvaptan, which was attributed to a lower incidence of urinary tract infections, stones and hematuria12. However, prevention of pain is not, by itself, an indication for tolvaptan at this time. Tolvaptan is discussed extensively in the ADPKD-specific treatment section.
Recommendations1. Bleeding:
- a
For symptomatic treatment of cystic hemorrhage it is suggested bed rest, analgesics, and in case of significant hematuria, sufficient hydration to increase diuresis to 2–3L per day (D).
- b
It is suggested to advise home self-treatment of macroscopic hematuria, following pre-established instructions in patients with previous episodes of similar characteristics. If the bleeding is severe or persistent, the patient should go to the Emergency Room (no degree of evidence).
- c
It is suggested that the following therapeutic options be considered depending on the magnitude and persistence of the hemorrhage: intravenous fluids, red blood cell transfusion in case of anemia, desmopressin if the eGFR is <15ml/min/1.73m², bladder catheterization if there are clots with the hematuria to prevent obstruction of the urethra and, in severe active bleeding, percutaneous embolization of the bleeding artery or nephrectomy (D).
- d
A risk-benefit assessment should be performed when starting anticoagulants or antiplatelets in patients with a history of macroscopic hematuria (D).
- a
2. Infection:
- a
It is recommended to hospitalize the patient with symptoms of infection of renal or hepatic cyst (D).
- b
The diagnosis of a probable cystic infection can be based on the following criteria: a) fever >38°C; b) localized flank pain, and c) CRP>5mg/dl (D).
- c
Elevation of alkaline phosphatase and CA19.9 compared to baseline values suggest hepatic cyst infection (C).
- d
It is recommended to perform urine and blood cultures if urinary or cystic infection is suspected (D).
- e
In case of suspected cystic infection, is recommended empirical antibiotic therapy with lipid-soluble drugs with good penetration into cysts and activity against gram-negative enterobacteriaceae, such as quinolones. It is suggested to adjust the antibiotic treatment according to the evolution and sensitivity tests (D).
- f
The duration of antibiotic treatment should be 4–6 weeks (D).
- g
It is suggested to add a second antibiotic (cephalosporins or carbapenems) and perform imaging tests to reassess the presence of possible complications if no improvement is observed within the first 72h (D).
- h
Imaging tests are recommended to locate the infected cyst in case of poor evolution and the need to perform invasive procedures. It is suggested to perform PET/CT if the infected cyst has not been located using other techniques (D).
- i
It is suggested to consider invasive procedures if the infected cyst has been identified through imaging tests and there is no response to antibiotics. Invasive procedures may include percutaneous or surgical drainage if the cyst diameter is >3–5cm, and nephrectomy for emphysematous cysts, recurrent infections, transplant candidates with recent refractory cystic infection, or staghorn calculus causing recurrent urinary tract infections in the presence of poor or absent kidney function (C).
- j
Complications such as urinary tract obstruction, perirenal abscess, or urolithiasis should be ruled out if fever recurs after discontinuation of antibiotics. If these complications are not identified, it is suggested to prolong antibiotic treatment, even several months, in order to eradicate the infection (D).
- a
3. Lithiasis:
- a
Noncontrast CT is recommended to confirm the diagnosis of nephrolithiasis (B).
- b
In case of nephrolithiasis, it is recommended a metabolic study (C). It is suggested to consider oral potassium citrate in patients with urolithiasis and hypocitraturia (C).
- c
It is suggested to consider urine alkalinization in uric acid lithiasis (C).
- d
The indication for percutaneous nephrolithotomy and extracorporeal shock wave lithotripsy must be individualized (D).
- a
4. Chronic pain:
- a
It is suggested to evaluate the presence of chronic pain and identify and treat, if possible, the cause (D).
- b
It is suggested symptomatic treatment of mechanical musculoskeletal pain or secondary to increased kidney size is (D).
- c
Opioid analgesics are suggested to be reserved for episodes of acute pain (D).
- d
It is suggested that invasive procedures be considered, such as a celiac plexus block or others, if the pain secondary to increased kidney or liver size is not controlled with medical treatment (D).
- a
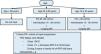
The course of ADPKD is highly variable and not all patients progress to ESRD. Renal survival is affected by non-modifiable factors such as age, gender, the affected gene and the type of mutation, and by modifiable factors such as hypertension, proteinuria and others5,73.
The mean age at the start of renal replacement therapy (RRT) in patients with ADPKD is lower than in patients without ADPKD, and survival is longer.
Kidney complications can persist even after advanced or end-stage CKD but rarely lead to serious problems. In cases of uncontrollable frank hematuria, infection of a cyst or a large renal volume, nephrectomy would be considered.
CV disease is the main cause of death73.
Among the different dialysis modalities, it has been suggested that peritoneal dialysis is a reasonable option, offering a better prognosis and quality of life to patients with ADPKD than to those who do not carry this disease as a cause of CKD74,75. This modality should not be denied due to the mere existence of cystic nephromegaly76. However, in patients with very large kidneys or livers, the lack of space may restrict the area available for peritoneal exchange and increase the chances of hydrothorax and abdominal hernias. In these cases, hemodialysis should be considered as the best option. The same could be said for those patients with recurrent diverticulitis.
Kidney transplantation in ADPKD is the best RRT option. It has similar results to other non-diabetic patients5,73 and ADPKD is not a risk factor for the development of post-transplant diabetes77.
The main difference lies in the need to assess the existence of ICA and to decide whether to perform nephrectomy of a native kidney78 to leave the necessary space for a kidney transplant. There is not enough evidence about whether nephrectomy of the native kidney should be performed before transplantation or simultaneously in the act of transplantation79. Laparoscopic nephrectomy is a good option, as long as the center has experience in this type of surgery.
Preventive kidney transplantation from a living donor shows a better evolution.
Although the use of mTOR inhibitors has been suggested to reduce TRV after kidney transplantation, there is insufficient evidence to recommend their use as first-line treatment in patients with ADPKD.
Recommendations- 1
Peritoneal dialysis and hemodialysis are valid RRT modalities for patients with end-stage CKD secondary to ADPKD (C).
- 2
Heparin should be avoided during hemodialysis in patients with frank and recurrent hematuria (C).
- 3
Kidney transplant is the recommended form of RRT. Living donor transplantation is a valid option to consider due to its better evolution (D).
- 4
Elective native nephrectomy should be considered before renal transplantation when kidney size precludes adequate graft placement and in symptomatic patients. Whether it is done before or during the transplant will depend on the experience of each center. (D)
- 5
Nephrectomy of a native kidney should also be considered in the event of complications such as neoplasia, hemorrhage, or persistent infection (D).
Polycystic liver disease is the most common extrarenal manifestation. It occurs in up to 90% of patients older than 35 years81. It is defined as the presence of at least 20 simple cysts in the liver. Liver cysts usually appear later than renal cysts and are very variable in size.
The main risk factors for the development of liver cysts are age, female gender, multiple pregnancies, the use of estrogens, hormone replacement therapy or contraceptives83. Liver volume tends to decrease or not increase in women after 48 years of age, thus highlighting the importance of the hormonal role in the development of cysts81,82.
Most patients remain asymptomatic and only a small proportion (20%) develop massive liver disease. The main symptomatology derives from hepatomegaly, which can cause extrinsic compression of the thoracic and abdominal organs: abdominal distension, abdominal pain, gastroesophageal reflux, early satiety, nausea and vomiting, dyspnea, orthopnea, hernias, uterine prolapse, rib fractures, malnutrition, loss of muscle mass, back pain, venous obstruction (hepatic, inferior vena cava, portal), bile duct obstruction and others. Abdominal pain is usually the most frequent manifestation. The liver parenchyma is preserved despite the growth of liver cysts, so it is rare that they cause liver failure. Bile duct involvement is a serious complication that can occur in patients with ADPKD and must be taken into account in all patients with abdominal pain. It is more common in males. Bile duct dilation has been described in 17–40% of patients84.
The biochemical alterations that we can be observed are: elevation of alkaline phosphatase and GGT. Bilirubin levels are usually normal but can be elevated by compression of the bile duct by a cyst. CA19.9 levels are elevated in up to 45% of patients with polycystic liver disease and correlate with liver volume83 and has been proposed as a biomarker of hepatic cyst infection, since its levels tend to rise during this process and fall with its resolution66. It can also be elevated: CA125, CEA and AFP.
Early detection of polycystic liver disease does not provide advantages over a possible therapeutic intervention since only severe symptomatic cases are treated.
The indication for treatment is established when the patient presents severe symptoms, most of them derived from the compression of adjacent structures by hepatic growth. Therefore, the goal of treatment is to reduce liver volume.
Women should avoid taking oral contraceptives or hormonal therapy with estrogens to prevent the growth of cysts.
There are options of medical and surgical treatment. The interventional treatment options and the results are summarized in Table 6. Regarding medical treatment, somatostatin analogs (octeotride and lanreotide) are the only drugs that have been shown to modify the natural course of the disease. According to clinical trials somatostatin analogs may reduce liver volume by 6% reduction during 1–3 years85–87, but studies are needed to demonstrate long-term efficacy.
Interventional treatment of complications derived from polycystic liver disease.
| Method | Indication | Result | |
|---|---|---|---|
| Aspiration-sclerotherapy | Cyst aspiration and subsequent administration of a sclerosing agent (ethanol the most frequent) | Dominant cyst (greater than 5cm) responsible of the symptoms | In 70% improves or makes symptoms disappear |
| Other agents used: minocycline or tetracycline | Cyst regression: 22% total/19% partial | ||
| Causes destruction of the epithelial lining of the cyst | Recurrences up to 21% | ||
| Few side effects. The most frequent: abdominal pain due to peritoneal irritation during the instillation of ethanol | |||
| Fenestration | Combines aspiration with resection of the superficial walls of the cysts | Patients that do not respond to aspiration-sclerotherapy | Reduces the severity of symptoms in 92% cases, |
| Two types: 1) laparoscopic: preferred due to less associated complications; 2) open | Recurs in 24% and | ||
| 23% present complications: ascites, pleural effusion, arterial or venous bleeding | |||
| Predictive factors for poor outcome: previous surgery, diffuse cystic disease, deep cysts | |||
| liver resection | Resect the most affected liver fragment | Severely affected with at least some unaffected liver segment | Effective in 86% |
| It is usually combined with fenestration in the area that is not resected | Complication rate rises up to 50%: ascites, hemorrhage, bleeding | ||
| Mortality rate: 3% | |||
| May complicate a future liver transplant | |||
| Liver transplant | Liver transplant | Very severe involvement and complications difficult-to-treat | The only curative treatment |
| Sometimes conbined liver-renal transplant | Five year survival: 92% | ||
| Other options | Embolization of branches of hepatic artery | Little experience |
In animal models, a new somatostatin analog, pasireotide, is more powerful and effective than the current ones88. In general, they are well tolerated, but lose their efficacy after discontinuation. At the moment, they have not been approved by the EMA and can only be used in the context of a clinical trial or for compassionate use in highly symptomatic patients89.
The mTOR inhibitors have not been shown to be effective alone or in combination with somatostatin analogs. Ursodeoxycholic acid has been investigated as another possible therapeutic alternative, without definitive results so far. TGR5 antagonists are also being investigated84.
The efficacy of tolvaptan on liver cysts is not well characterized. It could act on the V2 receptors of the cholangiocytes, reducing the levels of AMPc and, therefore, the proliferation of the cysts. There are only 2 cases in the literature in which tolvaptan reduced liver size.
Polycystic liver disease can cause acute complications, such as cystic infection or bleeding. The infection is clinically characterized by abdominal pain in the right hypochondrium together with fever. Generally they are monomicrobial. The most common germs that cause infection are gram-negative bacilli (eg, Escherichia coli). Its route of entry is retrograde through the bile duct. Laboratory tests revealed leukocytosis, elevated erythrocyte sedimentation rate, CRP, elevated bilirubin, liver enzymes (GOT, GPT), GGT, and alkaline phosphatase.
The determination of CA19.9 has been proposed as a biomarker of hepatic cyst infection since its levels tend to rise during this process and fall with its resolution66.
Diagnosis is usually made by CT or MRI, but the most sensitive diagnostic tool for cystic infection is positron emission tomography after administration of 18-fluorodeoxyglucose (PET-FGD)58,90.
Intracystic bleeding is rare. Symptoms can be very similar to those of an infected cyst, although fever is rare. Abdominal pain is usually more intense. Diagnosis is made by CT or MRI. Treatment is based on the administration of analgesics.
Cyst rupture is rare and can cause acute abdominal pain and ascites. Treatment is symptomatic.
Another very rare complication is recurrent episodes of cholangitis91.
Recommendations- 1
Patients with moderate-severe polycystic liver disease should avoid taking estrogens and drugs that stimulate cAMP accumulation (eg, caffeine) (D).
- 2
In patients with mild polycystic liver disease with hormone replacement therapy, the minimum effective dose should be administered and, if possible, by transdermal route (D).
- 3
If hepatic cystic infection is suspected, a CT should be performed. The treatment of choice is the administration of antibiotics (quinolones) for at least 6 weeks. If fever persists 72h after starting antibiotic treatment, a 3rd generation cephalosporin should be associated. When signs of infection persist after 3–5 days of starting treatment, a PET-FGD is recommended to try to locate the infected cyst, if it was not located with CT or MRI. Percutaneous drainage of the cyst under radiological control is only recommended if signs of infection persist, to identify the causative agent (D).
- 4
Intracystic hemorrhage should be diagnosed with MRI and should be treated with analgesics (D).
- 5
Treatment of polycystic liver disease is only indicated in highly symptomatic patients. The goal is to reduce liver volume. Polycystic liver surgery requires an expert surgeon, given the abnormal liver anatomy and the high morbidity of surgical procedures in these cases (C).
The prevalence of ICA in patients with ADPKD is around 8–12%, 5 times higher than that of the general population92–97. The mean age at rupture is 41 years; about 10 years earlier than in the population without ADPKD97. The most determining risk factor for having an ICA is a positive family history for ICA and/or subarachnoid hemorrhage (SAH), which is associated with a prevalence of 20–29% compared to 6–9% in those without a history94–97.
Clinically, ICA of ADPKD are generally asymptomatic, 85% are located in the anterior circulation and the measure is less than 7mm, and may be multiple in 20% of cases. The rupture of ICA causes SAH, the most serious complication of ADPKD. It is characterized by the appearance of sudden and intense headache, sometimes accompanied by loss of consciousness, it may cause death (30–60%) or severe disability (30%)98,99. The risk of rupture correlates with the size of the aneurysm and with the existence of a family history of ICA and/or HSA, but also with the location, the presence of an aneurysmal sac, tobacco consumption, the existence of hypertension, cocaine, the use of estrogens or anticoagulants92,96,98,100,101. Death due to neurological causes accounts for 11% (6% aneurysm rupture, 5 % cerebral hemorrhage) of the causes of death in ADPKD, behind cardiac causes (36%) and infections (24%). The risk of intracranial hemorrhage is 3 times higher in patients with ADPKD on RRT than in non-ADPKD, and the greatest difference is seen in patients on hemodialysis.
Gadolinium-free MRI is the technique of choice for diagnosing ICA as it avoids the iodinated contrast of CT angiography. There is agreement on the indication of preventive screening in patients with a family or personal history of ICA or SAH14,96,101. The rate of de novo aneurysm formation in patients with negative prior screening has been estimated at 0.32 (95% CI, 0 to 0.68) per 100 patient-year96. In patients with a positive family history and negative results, it is recommended to repeat the screening after 5 or 10 years96,101,102.
The treatment of ICA should be assessed by multidisciplinary teams with neurosurgeons and interventional radiologists from experienced centers. If cause symptoms they should be treated; asymptomatic patients always require follow-up and, depending on the case, intervention based on their characteristics (Table 7). The PHASES score is useful to assess the risk of rupture103,104.
Management of ntracranial aneurysms (ICA) in ADPKD.
| Indications for preventive detection, screening, of ICA in patients with ADPKD |
| - Family or personal history of subarachnoid hemorrhage and/or aneurysm |
| - Symptoms suggestive of aneurysm |
| - Work or activity in which loss of consciousness can be lethal |
| - Preparation for major elective surgery |
| - Extreme anxiety of the patient in relation to the risk of having an aneurysm |
| Management of asymptomatic ICA without rupture (modified from Williams and Brown RD, Neurol Clin Pract. 2013) |
| A) Treatment is recommended: |
| - ICA≥12mm in diameter |
| - Symptomatic ICA |
| - ICA that changes and increases the size |
| B) Treatment may be considered: |
| 1) AIC 7–12mm in diameter that meet any of the following characteristics: |
| - Young patient |
| - High-risk location (posterior circulation or posterior communicating artery) |
| - Saccular aneurysm |
| -Family history of subarachnoid hemorrhage |
| 2) ICA<7mm in diameter in young patients who meet any of the following characteristics: |
| - High-risk location (posterior circulation or posterior communicating artery) |
| - Saccular aneurysm |
| - Family history of subarachnoid hemorrhage |
| C) Treatment is not recommended: |
| - ICA<7mm in diameter in the anterior circulation without a family history of subarachnoid hemorrhage and non-saccular characteristic |
| - Cavernous aneurysm of the internal carotid artery |
The type of intervention must be personalized, it may consist of surgical clamping or the use of an intravascular coil.
ICA: intracranial aneurysm.
Conservative treatment is desirable in polycystic patients with aneurysms <7mm of the anterior circulation. Although the risk of growth is low, initial radiological follow-up is recommended every 3–6 months, delaying it to annual and every 2–3 years once its stabilization is observed2,94,95,105. Treatment is not innocuous and iatrogenic events are more frequent in ADPKD than in the general population, both for neurosurgery (11.8% vs. 6.4%) and for interventional radiology (9.4% vs. 3%)106.
It is important not to forget the modifiable factors to reduce the risk of the appearance and growth of aneurysms in the general population and in ADPKD (smoking cessation, BP control and CV risk).
In general, universal screening for ICA is not recommended14,96,101,105 and it is indicated only in cases with a family or personal history, professions with risk, preparation for major elective surgery and patient anxiety despite adequate information (Table 7). Kidney transplantation is a major elective surgery, however there is no agreement on prior screening for ICA.
The indication, timing and frequency of screening for aneurysms is a matter of debate. Some authors propose universal screening based on the difficulty of obtaining a good family history, the non-negligible presence of aneurysms in cases with no history, and a higher rate of rupture in some series than the usually described108. The decision to perform universal screening depends on the accessibility of screening methods, the incidence of ICA and its rupture, the consequent morbidity and mortality, the possible therapeutic actions and their results. The selection of the groups studied is also decisive, and it is possible that in some subpopulations such as the Japanese and Finnish the risk is higher96,101.
Flahault et al.96 exhaustively review their experience at the Mayo Clinic and the information available, analyzing with particular attention the rate of aneurysm rupture per 100 patient-years in published series94,95,102,107 and in a group made up of 6,095 polycystic patients from clinical trials, without image screening, with clinical follow-up of 19,400 patient-years.The study confirms the importance of family history, highlighting the history of SAH as having more importance than the presence of ICA, although the majority (>60%) of patients with ICA did not have a family history. The rate of aneurysm rupture, in patients without prior screening, is 0.04 per 100 patient-years (95% CI, 0.01 to 0.06) and the indication for screening is reaffirmed, restricted to cases with family history according to Table 7. However, clinical trials usually recruit patients with more preserved kidney function and both the incidence of ICA and SAH increases with the progression of kidney disease, being higher in haemodialysis. Thus, in the REPRISE trial, which included patients with poorer renal function, the incidence of rupture was 0.19 per 100 patient-years (95% CI, 0 to 0.40).
Recommendations- 1
The most suitable examination to detect ICA is MR angiography without gadolinium. If this is not possible, CT angiography is an acceptable alternative (C).
- 2
Preventive detection of ICA should be performed only in the situations indicated inTable 7(D).
- 3
Asymptomatic ICA should be evaluated in collaboration with neurosurgery and interventional radiology according to the guidelines shown inTable 7(C).
- 4
Urgent brain CT is indicated if a patient with ADPKD develops severe acute headache with or without loss of consciousness (C).
- 5
All symptomatic aneurysms should be treated (C).
- 6
The type of ICA treatment should be decided in a personalized multidisciplinary session and may include surgical clamping of the aneurysm neck or endovascular treatment with a platinum coil(C).
Most of the clinical manifestations (renal and extrarenal) of ADPKD occur during adulthood. However, the disease is already present at birth and can be diagnosed in children, newborns and even fetuses109.
The KDIGO Guidelines14 and the international radiological consensus for cystic kidney diseases in children110 address the diagnosis of ADPKD in Pediatrics:
–There are no specific radiological criteria for children. The Pei criteria were derived from patients older than 15 years and have low sensitivity in younger children.
–In children under 15 years of age with a positive family history, the presence of a renal cyst, nephromegaly, or both should be considered highly indicative of ADPKD. Routine genetic testing is not recommended, since there is no specific treatment, directed at the pathogenesis of cysts, approved in children (see indications for genetic testing).
–In the absence of a family history of ADPKD, and also in the absence of other data suggestive of a different cystic disease, the presence of large kidneys with cysts is indicative of ADPKD. An ultrasound should be performed on the parents and grandparents (especially if the parents are under 40 years old) and if they do not have the disease, a genetic study is recommended.
In ADPKD, large, hyperechoic, and even cystic kidneys may (although not necessarily) be seen prenatally. However, the main cause of prenatal bilateral renal hyperechogenicity is abnormalities in the HNF1B gene111.
In pediatric ADPKD we distinguish 2 presentations:
- 1
Children with early symptoms (less than 2% of children): large, hyperechoic kidneys with multiple cysts are already evident in prenatal ultrasounds. Clinically, they present renal failure and hypertension in the first months-years of life and may be clinically indistinguishable from autosomal recessive polycystic kidney disease. This includes children with Potter sequence and significant perinatal/neonatal morbidity and mortality. Severe and early forms of ADPKD are usually caused by mutations in multiple genes causing cystic kidney disease, and hypomorphic PKD1 alleles 33 may occasionally be involved (seesection on genetic diagnosis). In general, children with enlarged kidneys on ultrasound tend to have more clinical manifestations than children with normal-sized kidneys109.
- 2
Asymptomatic children with early diagnosis: here we would include children with a family history of ADPKD and who present a cyst in the prenatal ultrasound or in the first months of life. The vast majority of these children will be asymptomatic throughout their childhood years and will present with CKD in adulthood. However, it has been suggested that they have a worse prognosis than those diagnosed later in life109,112,113.
Most affected children have few or no symptoms during childhood, although some may develop renal cysts, hypertension, macroscopic hematuria, abdominal pain, abdominal mass and, less frequently renal failure112,114–116. Low birth weight per se may predispose to an earlier onset of ESRD in patients with ADPKD117.
Routine ultrasound screening of asymptomatic children with one parent affected by ADPKD remains controversial, given that there is no specific approved treatment in children under 18 years of age and, furthermore, a normal ultrasound result could be falsely reassuring. It is desirable not to carry out unnecessary examinations but since ADPKD begins in childhood, it is important to identify early risk factors for disease progression that can provide us with the greatest potential for effective early intervention. The main one is hypertension, which, although infrequent, may appear in childhood.
A prevalence of hypertension is estimated at around 20% in children and young adults (up to 21 years of age) with ADPKD118. The ABPM indicates a rate of HTN of 31, 42, and 35% during the day, night, or 24h, respectively. At the time of ABPM determination, 95% of the children had normal renal function119. These percentages are probably overestimated due to a severity bias in the pediatric cohorts. There is evidence that in children with ADPKD, borderline hypertension can increase left ventricular hypertrophy, reinforcing the need for optimal control120.
The ACE inhibitors offer a potential benefit for reduce CV function deterioration and the loss renal function over time in children with borderline hypertension (BP inthe 75th and 95th percentile)121.
Extrarenal manifestations are exceptional during the pediatric age.
The recommendations in the pediatric age are based on a fundamental fact: we do not have a specific treatment (aimed at the pathogenesis of the cysts) approved in children. The recommendations will be different when we have a specific treatment approved for children.
Recommendations- 1
Screening for ADPKD in children of polycystic parents is questionable from an ethical point of view since there is no absence of a specific treatment (no degree of evidence).
- 2
An imaging study should be performed in children of polycystic parents who present hypertension or hematuria (no degree of evidence).
- 3
A genetic study should be carried out in children with very early and severe manifestations; this is to assess the contribution to the phenotype of other genes involved in renal cystic diseases (D).
- 4
All children with ADPKD with symptomatic disease should be followed by a pediatric nephrologist (D).
- 5
Every child of a patient with polycystic disease should have their BP checked at each routine visit with their pediatrician (D).
- 6
If hypertension is detected, treatment with ACE inhibitors should be started (no degree of evidence).
- 7
Routine evaluation of extrarenal manifestations in childhood is not advised (no grade of evidence).
In 2015, the EMA approved the use of tolvaptan, a vasopressin V2 receptor antagonist, expressed mainly in the distal nephron and collecting duct, to slow down cystic development and the progression of kidney disease. This medication is indicated in adult patients with ADPKD in CKD stages 1–4 with evidence of rapid progression (see section Recommendations for assessment in ADPKD progression). Tolvaptan decreases cAMP levels in cystic cells and reduces fluid secretion into the cyst as well as cell proliferation. We cannot exclude the possibility that tolvaptan has other additional mechanisms of renoprotection, through effects on renal hemodynamics (glomerular hyperfiltration or anti-inflammatory effect). The goal of tolvaptan treatment is to maintain an inhibition the action of vasopressin on V2 receptors. Tolvaptan administration should always be associated with classic renoprotection measures such as changes in lifestyle, BP control, preferably with RAAS blockers.
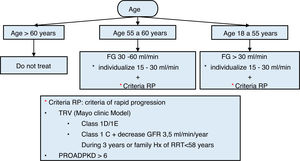
Several randomized studies with more than 1,000 patients have shown that tolvaptan reduces the increase in total renal volume (TRV), improving symptoms caused by TRV, such as pain. In the TEMPO 3:4 study (1445 patients aged 18–55 years with Ccr≥60ml/min and TRV≥750ml with a duration of 3 years) the growth of TRV was reduced by 49% and the drop in eGFR measured by the inverse of plasma creatinine was also reduceby 26% as compared to placebo12. An extension of this study, TEMPO 4:4, showed persistence of these positive effects, especially on eGFR, in such a way that the earlier start of tolvaptan was associated with better conservation of GFR122. In patients with more advanced CKD, stages 2–4 and aged between 18–65 years, the REPRISE study showed a 35% reduction in the drop of GFR after one year of treatment with tolvaptan (2.34 vs 3.61ml/min /1.73m² in the group treated with tolvaptan vs. placebo)13. Other studies have reported a sustained and cumulative effect of tolvaptan up to 11 years later123. The impact of tolvaptan on eGFR loss is, in percentage terms, similar to that of RAS blockade in other kidney diseases.
A careful selection must be made of those patients who are candidates to start treatment with tolvaptan, assessing contraindications, adverse effects and the patient’s lifestyle, so the decision must be made jointly (Assessment of rapid progression in ADPKD section).
Among its adverse effects, 65%–95% of patients who start treatment with tolvaptan present water diuresis122, so patients must have easy access to water and maintain a consistent liquid intake. As a consequence, some patients may present hypernatremia, so it is important to insist on the need to drink water when thirsty, including at night if they get up to urinate, and monitor this parameter during treatment. A variable percentage of patients are unable to tolerate polyuria and abandon treatment.
There is a special control call for idiosyncratic hepatotoxicity, rare, but with the possibility of serious liver damage. In order to monitor this effect, transaminases and liver function tests should be monitored monthly during the first 18 months of treatment and every 3 months after 18 months (http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002788/WC500187921.pdf).
There are clinical situations that must be taken into consideration when starting treatment with tolvaptan, such as the presence of a history of hyperuricaemia-gout, the use of diuretics, diabetes mellitus or urinary problems that prevent a high urinary flow. Such conditions can potentially be worsened by the use of tolvaptan. Tolvaptan (Jinarc®, Otsuka Pharmaceutical SA) should be given twice a day. The morning dose at least 30min before breakfast and the second dose 8h later with or without food. The dose regimen will be:
–45mg+15mg
–60mg+30mg
–90mg+30mg.
After the initial dose, the dose will be escalated until reaching the maximum of 120mg/day. This dose escalation and at the discretion of the nephrologist can be done between 1 and 4 weeks. Patients should be maintained on the highest tolerated dose of tolvaptan.
It is recommended to evaluate urinary osmolarity, in the first urine of the morning (before the morning intake) to confirm tolvaptan intake and the achievement of its objective in those patients at the beginning and follow-up of treatment with tolvaptan. A reduction below 275–295mOsm/kg is considered ideal in most cases, although in those patients in an early phase of ADPKD, it could be considered a reduction of up to 200 mOsm/kg, more sensitive to aquaretic effects of tolvaptan. There is controversy about titrating the dose of tolvaptan based on urine osmolarity. According to American recommendations124, the goal of treatment would be to achieve a urinary osmolarity of less than 280mOsm/l in the first morning urine, before the morning dose. Therefore, if this is achieved with the minimum dose of tolvaptan, it would not be necessary to continue escalating the dose, facilitating tolerance. However, although it seems reasonable to look for ways to individualize the dose, several considerations must be made and in fact Chebib et al.124 suggest that more studies are needed in this regard:
- a
Urine osmolarity was not used to guide tolvaptan dosing in clinical trials, therefore, dose adjustment based on urine osmolarity has not been tested in a clinical trial and should be considered experimental.
- b
The goal of tolvaptan treatment is to maintain a continuous inhibition of the action of vasopressin on V2 receptors. If increasing the dose leads to a greater increase in urine osmolarity, this implies that the V2 receptors were not sufficiently blocked with the previous dose.
- c
In ADPKD there is a progressive defect in the ability to concentrate urine (partial resistance to ADH, partial nephrogenic diabetes insipidus), a phenomenon common to all advanced CKD but magnified in ADPKD by the presence of cysts that distort renal architecture and limit the ability to form an adequate corticomedullary osmolar concentration gradient. As a consequence, these patients may have high ADH levels despite, and precisely because, they have polyuria with low urine osmolarity. That is, in a given patient it may be difficult to distinguish low but not very low urine osmolarity because a reasonable degree of V2 receptor blockade has been achieved or because the patient has an inability to concentrate urine due to ADPKD and, as consequence, they have estimulation of ADH, with persistent activation of V2 receptors.
- d
The main argument in favor of adjusting the dose according to urinary osmolarity would be to facilitate clinical tolerance by limiting the aquaretic effect; the tolerance to aquaretic effects is usually lower when the eGFR is more conserved, since patients usually start with a smaller volume of diuresis (having not yet developed the inability to concentrate urine) and have a greater diuretic response, given their higher eGFR. In this sense, the discussion of the magnitude of the symptoms with the patient could guide the dose adjustment to improve tolerance.
- 1
Treatment with tolvaptan can be considered in patients with ADPKD up to 60 years of age and criteria of rapid progression in CKD stages 1–3b and exceptionally stage 4 (D).
- 2
It is recommended that the indication of the treatment be shared between the patient and the nephrologist once the patient’s clinical characteristics and lifestyle have been assessed (D).
- 3
It is recommended to take into account and assess adverse effects, especially hepatotoxicity during treatment with tolvaptan (D).
- 4
It is recommended to start treatment with tolvaptan with 45mg in the morning and 15mg after 8h of the morning dose and increase the dose until reaching 90/30mg as the highest tolerable dose (D).
- 5
It is recommended to evaluate the osmolarity urine in the first urine in the morning, at the beginning and follow-up of treatment with tolvaptan to confirm therapeutic compliance and its efficacy. (D)
Tolvaptan is the only ADPKD medication that modifies progression of the disease and it approved by the EMA for ADPKD. Based on the aforementioned clinical variability and the inclusion criteria of clinical trials, the current indication for tolvaptan according to the EMA is “The treatment of adults with ADPKD G1-G4 with evidence of rapid progression of the disease” (https://www.ema.europa.eu/en/documents/product-information/jinarc-epar-product-information_en.pdf; accessed 21 Aug 2019). The EMA does not specify what it considers rapid progression. The EMA indication does not clarify what is considered as rapid progression.
To adequately define rapid progression, it is necessary to understand the clinical history of the disease, which, on average, is 6 decades. Thus, in ADPKD patients the renal function remains normal during the first decades of life despite the growth of the TRV. So at this stage it is especially useful to assess the TRV to determine the prognosis125. The clinical spectrum of polycystic disease is broad; there are patients who only develop a limited number of bilateral cysts, which are unlikely to lead to loss of renal function and require renal replacement therapy, while at the other extreme there are severe neonatal forms of the disease.
Therefore we recognize 2 situations:
- a
The patient who is already losing eGFR rapidly.
- b
The patient who is still in the initial stage of apparent stability of renal function during the first decades of life but with data that predict a rapid progression of CKD. These data may be a prediction of rapid progression based on genetic and clinical data or a finding that rapid progression is already occurring subclinically in the form of a rapid increase in TRV. The identification of risk factors for the rapid progression of CKD will make it possible to start early treatment in high-risk patients and start it before the stage of rapid loss of eGFR is achieved.
An additional problem is what is considered a rapid progression from the point of view of eGFR. There is a KDIGO consensus definition: loss of more than 5ml/min/1.73m²/year of eGFR. But this definition seems too strict for patients with ADPKD, which has a long natural history of disease (60 years). In fact, the mean GFR loss in the placebo group in the tolvaptan clinical trials was 3.6–3.8ml/min/year. To be practical, all those patients who need RRT before the average age of starting RRT in Spain, that is, around 65 years, can be considered that progresses rapidly.
The main risk factors for progression of CKD in ADPKD are genetic, VRT and hypertension. Genetic factors depend on the mutated gene (a pathogenic variant in PKD1 has a worse prognosis) and the type of sequence variant (the truncating type being more serious)126. VRT is the best predictor of CKD progression125,127. As renal volume increases, the rate of decrease in eGFR is greater125, although there are discordant results from clinical trials, where an improvement in TRV is not accompanied by an improvement in the evolution of eGFR128. HTN is associated with a higher TRV41.
The Working Group of Inherited Kidney Diseases (WGIKD) of the European Dialysis and Transplant Association (EDTA) together with the European Renal Best Practice Guidelines (ERBP) group proposed a definition of rapid progression in ADPKD that has now become obsolete as mentioned in their website (http://www.european-renal-best-practice.org/content/erbp-documents-topic; accessed 20 Aug 2019).
Based on the evidence provided by the TEMPO3/4, TEMPO 4/4 and REPRISE studies, we propose the following criteria to define rapid progression- 1
We suggest to stop using the EDTA/ERBP algorithm.
- 2
We suggest including patients up to 60 years of age in the assessment of rapid progression. The average age of ESRD in Spain is 65 years. We consider rapid progressors those patients with ADPKD who require RRT before the mean age of RRT in Spain (D).
- 3
We suggest to include, for assessment of rapid progression, patients with eGFR>30ml/min. At the individual level, treatment can be considered with eGFR>15ml/min (D).
- 4
We suggest that patients between 55 and 60 years should have a GFR<60ml/min at the start of treatment. If at this age the GFR is >60ml/min, it would be very unlikely for them to need RRT before the age of 65 (D).
- 5
We suggest that to assess the start of treatment with tolvaptan all patients should have a measurement of TRV by MRI or CT (or in cases with symptoms before the age of 35, although a genetic study showing a truncating mutation in PKD1 may be sufficient).
- 6
We suggest that a patient who meets one of the following criteria be considered a rapid progresser (Figs. 1 and 2), simplified algorithm:
- i
VRT adjusted for height, in patients with bilateral symmetric involvement, belonging to the Mayo classification 1D or 1E (C) or
- ii
PRO-PKD score>6 (C) or
- iii
Height-adjusted VRT in patients with bilateral symmetric involvement belonging to the Mayo 1C classification:
- a
If the retrospective decline in eGFR over the past 3 years is ≥3.5ml/year (corresponding to the average decline in eGFR in patients in Mayo Clinic class 1D) or
- b
If most of the relatives reached ESRD before 58 years (the mean age of RRT in Europe for ADPKD) (D).
- a
- i
- 7
When defining eGFR decline, we must take into account the following considerations (D).
- i
There are medications that modify the eGFR, including NSAIDs, ACE inhibitors, ARBs, and fenofibrate that must be taken into account (B).
- ii
Other causes besides ADPKD that justify a drop in eGFR (C) must be ruled out.
- i
- 8
When defining the eGFR, it must be taken into account the following considerations (D):
- i
In routine clinical practice, eGFR is used based on serum creatinine (CKD-EPI equation or MDRD4 in case of GFR<60). However, this is just an estimate that only applies to people who have an expected muscle mass for their gender or age.
- ii
The equation is wrong and its use is not recommended for people who have more muscle mass than expected (eg athletes) or less (eg malnutrition).
- iii
If it is suspected that the eGFR formulas cannot be applied to a particular patient, there are alternatives:
- -
24-h urine creatinine clearance.
- -
Measure eGFR with iohexol clearance.
- -
Cystatin C measurement.
- -
- i
- 9
We suggest that the VRT be evaluated by MRI (C).
- i
Currently, gadolinium-free MRI is the gold standard for measuring TRV when both kidneys are enlarged. The use of the ellipsoid formula is acceptable to measure the VRT. The distinction between “typical” and “atypical” is recommended with respect to renal morphology (B).
- ii
In patients with kidneys >13cm in diameter by ultrasound, we suggest to assess: based on age (slightly enlarged kidneys in young adults may indicate rapid progression while this is not the case in older ages) and renal appearance (eg, a large renal cyst is not significant but multiple cysts that increase renal size are). Perform a baseline MRI and repeat every 3–5 years if the patient is not receiving treatment with tolvaptan (D).
- iii
Serial TRV measurements are not recommended in routine clinical practice if renal diameter is normal or, during treatment with tolvaptan. Although they may be of interest in specific clinical situations (D). Centers that do not have access to MR can:
- a
Refer the patient to a center with an ADPKD unit.
- b
Use the PRO-PKD if the patient has presented urological symptoms or HTN before the age of 35.
- c
Perform a CT once and evaluate according to the Mayo classification.
- a
- i
The authors do not declare any conflict of interest. Otsuka Pharmaceutical has not been involved in the development of the guidelines. Their participation has consisted exclusively of financing their layout.
The Spanish clinical guidelines for ADPKD are endorsed by the Spanish Society of Nephrology (SEN), the Spanish Network for Renal Research (REDinREN RD016/0006)) and the patient association: Association for Research and Information on Hereditary Renal Diseases (AIRG -AND).
Special thanks are expressed to the Spanish nephrologists who have contributed their corrections to this consensus document: Rafael J. Esteban de la Rosa (Virgen de la Nieves Hospital, Granada) on behalf of GEEPAD; Eduardo Parra Moncasi (Miguel Servet Hospital, Zaragoza); Francisco Roca Oporto (Hospital de Poniente, Almeria); Jonay Pantoja (Hospital Peset, Valencia), and Ramón Saracho (Hospital de Galdakao, Bilbao).