Colistimethate sodium (CMS) treatment has increased over the last years, being acute kidney injury (AKI) its main drug-related adverse event. Therefore, this study aimed to evaluate the incidence and risk factors associated with AKI, as well as identifying the factors that determine renal function (RF) outcomes at six months after discharge.
Materials and methodsThis retrospective study included adult septic patients receiving intravenous CMS for at least 48h (January 2007–December 2014). AKI was assessed using KDIGO criteria. The glomerular filtration rate (GFR) was estimated by the 4-variable MDRD equation. Logistic and linear models were performed to evaluate the risk factors for AKI and chronic kidney disease (CKD).
ResultsAmong 126 patients treated with CMS; the incidence of AKI was 48.4%. Sepsis–severe sepsis (OR 8.07, P=0.001), sepsis–septic shock (OR 42.9, P<0.001), and serum creatinine (SCr) at admission (OR 6.20, P=0.009) were independent predictors.
Eighty-four patients survived; the main factors for RF evolution at the 6-month follow-up was baseline eGFR (0.58, P<0.001) and at discharge (0.34, P<0.001). Fifty-six percent (34/61) of the patients that developed AKI survived. At six months, 32% had CKD.
ConclusionsThe development of AKI in septic patients with CMS treatment was associated with sepsis severity and SCr at admission. Baseline eGFR and eGFR at discharge were and important determinant of the RF at the 6-month follow-up. These predictors may assist in clinical decision making for this patient population.
El tratamiento con colistimetato de sodio (CMS) se ha incrementado, siendo su principal complicación el fracaso renal agudo (FRA). El objetivo de este estudio fue determinar la incidencia de FRA y los factores de riesgo asociados, así como identificar los factores que determinan la función renal (FR) a los 6 meses del alta hospitalaria.
Materiales y métodosEstudio retrospectivo que incluyó pacientes adultos sépticos que recibieron CMS intravenoso durante al menos 48h (enero 2007-diciembre 2014). El diagnóstico de FRA se realizó según los criterios KDIGO. Se estimó el filtrado glomerular (FG) mediante la ecuación del MDRD-4. Se realizaron modelos logísticos y lineales para evaluar los factores de riesgo para el desarrollo de FRA y enfermedad renal crónica (ERC).
ResultadosCiento veintiséis pacientes fueron incluidos; la incidencia de FRA fue del 48,4%. Sepsis-sepsis severa (OR: 8,07; p=0,001), sepsis-shock séptico (OR: 42,9; p<0,001) y la creatinina sérica (CRs) al ingreso (OR: 6,20; p=0,009) fueron predictores independientes de FRA. Ochenta y cuatro pacientes sobrevivieron; el determinante principal de la evolución de la FR a los 6 meses de seguimiento fue el FGe basal (0,58; p<0,001) y al alta (0,34; p<0,001). El 56% (34/61) de los pacientes que desarrollaron FRA sobrevivieron. A los 6 meses, el 32% desarrollo ERC.
ConclusionesEl desarrollo de FRA asociado al tratamiento con CMS se asoció con el grado de severidad de la sepsis y la CRs al ingreso. El FGe basal y al alta hospitalaria fueron predictores independientes de la FR a los 6 meses de seguimiento.
The exponential increase in infections by multidrug-resistant Gram-negative (MDRGN) bacteria is accompanied by high morbidity and mortality, which has made it necessary to broaden the horizon to new antibiotics or to reintroduce old ones.1 One of these antibiotics, colistin, which belongs to the class of polymyxins, appeared in the early 50s. However, Its use was interrupted due to its nephrotoxic nature and neurotoxic side effects.2 After its reintroduction in the form of colistimethate sodium (CMS) at the end of the last century, the reported rate of acute kidney injury (AKI) has varied between 0 and 45%, being mild and reversible in most of the cases.3,4 AKI development seems to be related to the administered dosage, the length of the therapy,4,5 the concomitant use of other nephrotoxic drugs,5 the diagnosis of sepsis or shock,5 or the presence of hypoalbuminemia.6 On the other hand, although it is known that AKI predisposes the chronic kidney disease (CKD) development,7 only one article has analyzed the evolution and prognosis of the renal function (RF) in the long term in patients that underwent CMS therapy.8
The objective of this study was to analyze the incidence of AKI according to Kidney Disease Improving Global Outcomes (KDIGO) criteria and to determine the associated factors for its development in patients who received intravenous CMS.
The secondary aims were to determine RF outcome and the development of CKD at six months of follow-up.
Material and methodsDesign of the studyA retrospective, cohort study was carried out at the Ramón y Cajal Hospital (Madrid, Spain) between January 2007 and December 2014. The study was approved by the Clinical Research Ethics Committee of our hospital and conducted in accordance with the Declaration of Helsinki. Given the minimal risk nature of the study, we were granted a waiver of informed consent.
The population studied included all patients ≥18 years old identified in the Pharmacy Department database as treated with intravenous CMS (Rhône-Poulenc Rorer, Neuilly-sur-Seine, France) for at least 48h. In those who received more than one cycle of CMS therapy, only the first one was considered for the analysis. In all cases the length of treatment, the daily dosage and the cumulative dosage were recorded.
The exclusion criteria were: having developed AKI before the administration of CMS, within the first 48h of beginning CMS and up after the 72h following the end of CMS treatment. In addition, those patients who were receiving renal replacement therapy (RRT) at the time CMS therapy initiation were excluded.
Demographic information, clinical and biochemical parameters of prognostic interest were recorded from the electronic medical records at our institution, and included: baseline co-morbidities (history of neoplasms, diabetes mellitus, peripheral vascular disease, chronic kidney disease, heart failure, history of kidney or liver transplant), site of infection, type of bacteria isolated in cultures, Charlson Comorbidity Index,9 severity of illness (using Acute Physiology and Chronic Health Evaluation II (APACHE II),10 and Sequential Organ Failure Assessment (SOFA)11 scores at the time of the infectious episode.
Sepsis was classified according to the SEPSIS II criteria.12 Patients with any acute organ failure were considered to have severe sepsis, while septic shock was defined when patients were on vasopressors despite fluid resuscitation.
In addition information about the concomitant use of other potentially nephrotoxic medications (aminoglycoside, vancomycin, non-steroidal anti-inflammatory drugs [NSAID]) and/or iodinated contrasts was also recorded.
Serum creatinine (SCr) levels were recorded, when available, within the six months before admission (baseline SCr), at admission, at the beginning of the CMS therapy, at the time of the AKI diagnosis, when the treatment was completed, and at discharge or death. Renal function (RF) was calculated using the estimated glomerular filtration rate (eGFR) as determined by the abbreviated Modification of Diet in Renal Disease (MDRD-4) equation.13 We recorded the need for renal replacement therapy (RRT), defined as the beginning of intermittent hemodialysis or continuous RRT at some point during the hospital stay.
We analyzed the length of in hospital stays, the situation at the time of discharge and the vital and functional evolution at the 6-month follow-up.
Dose and equivalent terminologyColistimethate sodium (CMS) is an inactive prodrug of colistin that changes in vivo to active colistin (colistin base activity [CBA]). This product is available in Europe, India and some other countries in vials of 1 and 2 million international units (MIU) and/or milligrams of CMS. In North and South America, the southeast of Asia and Australia, the vials contain 150mg of CBA. In order to make it easier to compare the studies published and to avoid the possibility of inducing mistakes when administering the drug, the first International Conference regarding polymyxins that took place in Prato, Italy in May 2013, suggested the need to provide the equivalences between doses14 (30mg of CBA equal 80mg and a 1MIU of CMS).
The CMS is administered via intravenous between 30,000 and 60,000IU/kg/day. In patients with a decreased RF, the dose was adapted to it.15
DefinitionsAKI was defined and staged according to the Kidney Disease Improving Global Outcomes (KDIGO) criteria16; due to the retrospective quality of the study urine output was not taken into consideration. Therefore, any increase greater than 0.3mg/dl of baseline SCr in less than 48h, or a 50% increase in baseline SCr for seven days was considered AKI.
CKD status at sixth month was considered when estimated GFR by a four-variable equation derived from the Modification of Diet in Renal Disease (MDRD) study was lower than 60ml/min per 1.73m2.13,17
Statistical analysisData is presented as mean and standard deviation (±SD), or as median, interquartile ranges (IQR). To compare discrete variables, we used the Chi-squared test (or Fisher's exact). To compare two continuous variables, we used the Student's test for independent samples, or the non-parametric Mann–Whitney test.
Potential risk factors for the development of AKI were identified through univariate logistic regression analyses for each of the variables. All the co-variables clinically relevant and those who had a P value <0.20 on univariate analyses were included in multivariate, backward, logistic models.
The variable “sepsis severity” was divided into three categories: sepsis, severe sepsis and septic shock; and was evaluated as categorical variable: sepsis–severe sepsis, and sepsis–shock septic.
The accuracy of the model was evaluated using the Hosmer–Lemeshow goodness-of-fit test and regarding the area under a receiver operating characteristic (ROC) curve.
To analyze the variables that best associated with RF at the 6-month follow-up, we used univariate and multivariate linear regression models, adjusting for relevant covariates. The factors associated with CKD development at the 6-month follow-up in the patients who developed AKI were evaluated through univariate logistic regression because the number of cases did not allow for a multivariate logistic analysis.
A two-tailed P-value <0.05 was considered significant. Statistical analyses were performed with SPSS version 21 (IBM Corp., Armonk, US).
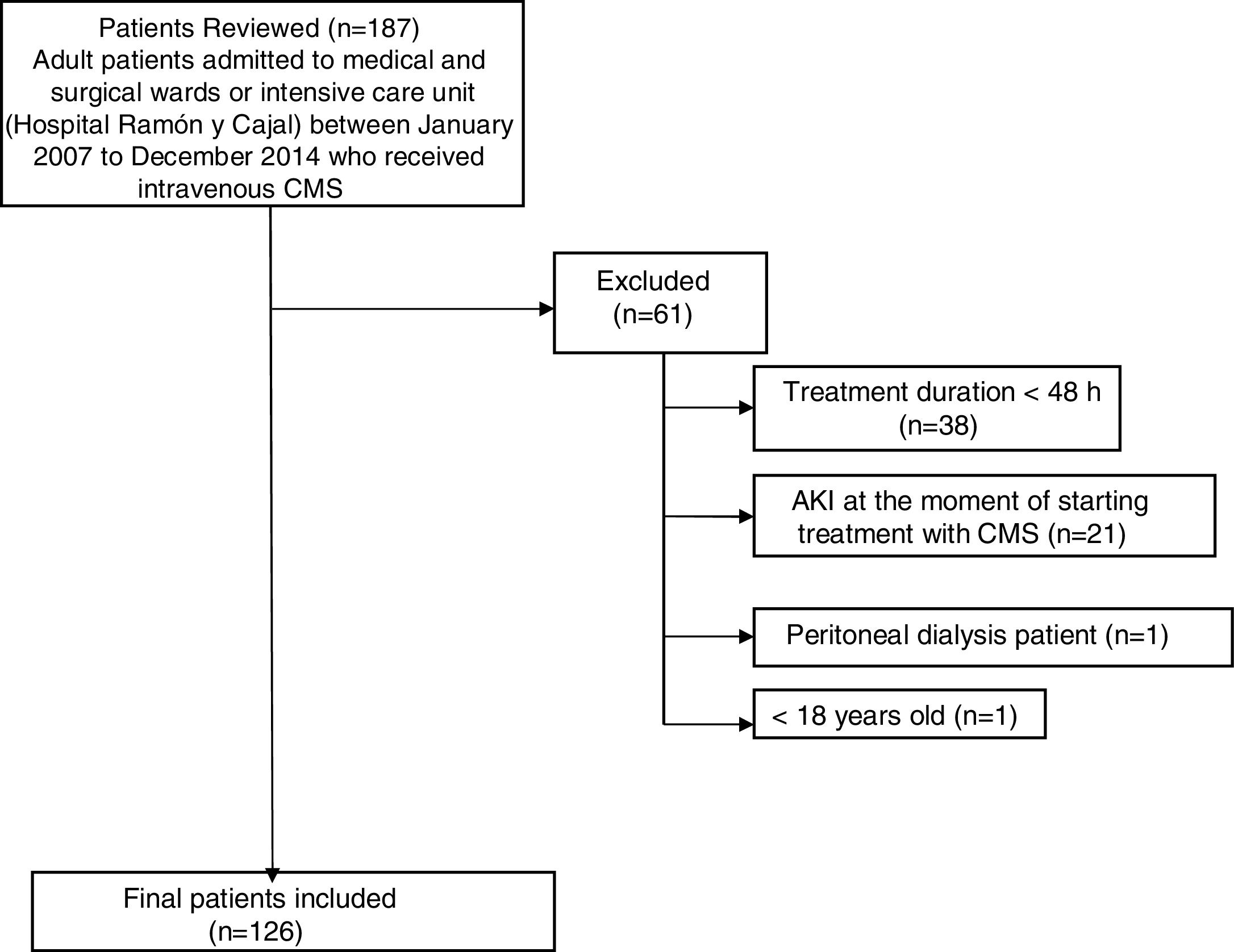
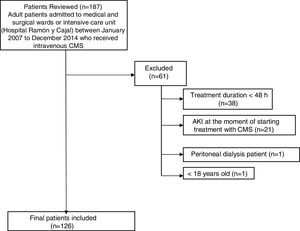
ResultsDuring the study period, a total of 187 patients were treated with CMS. Of these, 61 were excluded from the study (Fig. 1). Finally, 126 patients were included in the study. The general characteristics of the whole group of patients studied are presented in Table 1.
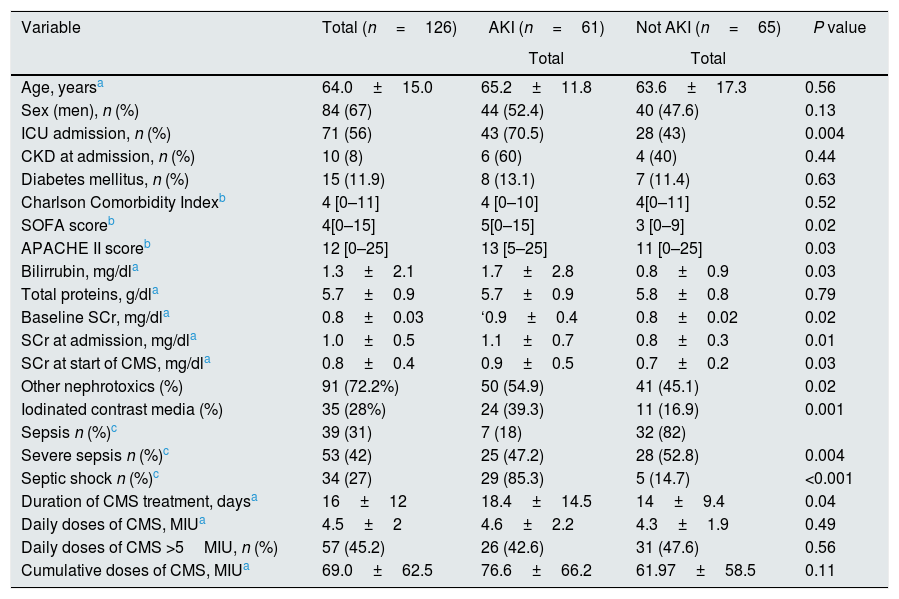
Clinical, demographic, and biochemical characteristics in the study cohort.
| Variable | Total (n=126) | AKI (n=61) | Not AKI (n=65) | P value |
|---|---|---|---|---|
| Total | Total | |||
| Age, yearsa | 64.0±15.0 | 65.2±11.8 | 63.6±17.3 | 0.56 |
| Sex (men), n (%) | 84 (67) | 44 (52.4) | 40 (47.6) | 0.13 |
| ICU admission, n (%) | 71 (56) | 43 (70.5) | 28 (43) | 0.004 |
| CKD at admission, n (%) | 10 (8) | 6 (60) | 4 (40) | 0.44 |
| Diabetes mellitus, n (%) | 15 (11.9) | 8 (13.1) | 7 (11.4) | 0.63 |
| Charlson Comorbidity Indexb | 4 [0–11] | 4 [0–10] | 4[0–11] | 0.52 |
| SOFA scoreb | 4[0–15] | 5[0–15] | 3 [0–9] | 0.02 |
| APACHE II scoreb | 12 [0–25] | 13 [5–25] | 11 [0–25] | 0.03 |
| Bilirrubin, mg/dla | 1.3±2.1 | 1.7±2.8 | 0.8±0.9 | 0.03 |
| Total proteins, g/dla | 5.7±0.9 | 5.7±0.9 | 5.8±0.8 | 0.79 |
| Baseline SCr, mg/dla | 0.8±0.03 | ‘0.9±0.4 | 0.8±0.02 | 0.02 |
| SCr at admission, mg/dla | 1.0±0.5 | 1.1±0.7 | 0.8±0.3 | 0.01 |
| SCr at start of CMS, mg/dla | 0.8±0.4 | 0.9±0.5 | 0.7±0.2 | 0.03 |
| Other nephrotoxics (%) | 91 (72.2%) | 50 (54.9) | 41 (45.1) | 0.02 |
| Iodinated contrast media (%) | 35 (28%) | 24 (39.3) | 11 (16.9) | 0.001 |
| Sepsis n (%)c | 39 (31) | 7 (18) | 32 (82) | |
| Severe sepsis n (%)c | 53 (42) | 25 (47.2) | 28 (52.8) | 0.004 |
| Septic shock n (%)c | 34 (27) | 29 (85.3) | 5 (14.7) | <0.001 |
| Duration of CMS treatment, daysa | 16±12 | 18.4±14.5 | 14±9.4 | 0.04 |
| Daily doses of CMS, MIUa | 4.5±2 | 4.6±2.2 | 4.3±1.9 | 0.49 |
| Daily doses of CMS >5MIU, n (%) | 57 (45.2) | 26 (42.6) | 31 (47.6) | 0.56 |
| Cumulative doses of CMS, MIUa | 69.0±62.5 | 76.6±66.2 | 61.97±58.5 | 0.11 |
AKI: acute kidney injury; APACHE: Acute Physiology and Chronic Health Evaluation; CKD: chronic kidney disease; CMS: colistimethate sodium; ICU: intensive care unit; MIU: million international unit; SOFA: Sequential Organ Failure Assessment; SCr: serum creatinine level.
The most common comorbidities found on the study patients were malignancy (16.6%), and chronic lung diseases (16%). The main infections for which CMS were administered were pneumonia (46%), followed by intrabdominal infections (18.3%), and urinary tract infection (13.5%). The most common isolated MDRGN microorganisms were Pseudomonas aeruginosa in 80 patients (63.5%) and Klebsiella pneumoniae in 22 (17.5%), whereas 38 patients (29.4%) had polymicrobial infections.
Incidence of acute kidney injury and risk factorsSixty-one of the 126 patients of the group (48.4%) developed AKI in a mean period of 13±12 days from the initiation of CMS therapy. According to KDIGO criteria, 23 patients (37.7%) reached stage 1; 20 patients (32.8%) stage 2 and 18 cases (29.5%) stage 3.
AKI developed more frequently in patients hospitalized in the intensive care unit (ICU). Patients with AKI differed significantly from patients without it in the following parameters: SOFA and APACHE II scores, bilirubin concentration, SCr at hospital admission and the beginning of CMS therapy, concomitant use of other nephrotoxic drugs and use of radiologic iodinated contrasts (Table 1).
All the patients studied had sepsis and the frequency of AKI increased according with the severity of sepsis: 18% of septic patients (18%) presented AKI, whereas this complication was present in 47% of cases with severe sepsis, and 85% of cases with septic shock (Table 1).
The length of CMS therapy was longer in patients who eventually developed AKI, as compared to the rest of study patients. In 8 patients (6.3%), CMS therapy was discontinued due to the development of AKI; none of them had a previous history of CKD. Twelve patients (9.5%) with normal RF at baseline received renal replacement therapy (RRT), 7 of then (58%) died at discharge. Of the 5 patients who survived, AKI progressed to CKD in 4 patients.
There were no statistically significant differences between patients with and without AKI regarding the rest of the studied variables (Table 1).
Neither the mean daily dose of CMS or the daily dose of CMS administered higher than 5MIU, nor the total dose of CMS were associated with the development of AKI (Table 1).
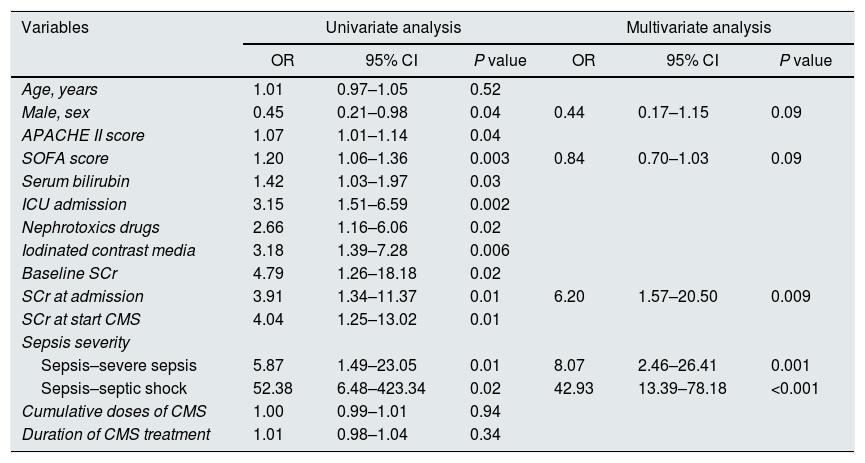
Independent predictors of AKI in multivariate logistic regression models (Table 2) included the SCr levels at admission (odds ratio [OR] 6.20; 95% CI=1.57–20.50, P=0.009) and the severity of sepsis (sepsis–severe sepsis (OR 8.07; 95% CI=2.46–26.41; P=0.001), sepsis–septic shock (OR 42.93; IC 95%=13.39–78.18; P<0.001).
Independent risk factors associated with the development of AKI: multivariate logistic regression.
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Age, years | 1.01 | 0.97–1.05 | 0.52 | |||
| Male, sex | 0.45 | 0.21–0.98 | 0.04 | 0.44 | 0.17–1.15 | 0.09 |
| APACHE II score | 1.07 | 1.01–1.14 | 0.04 | |||
| SOFA score | 1.20 | 1.06–1.36 | 0.003 | 0.84 | 0.70–1.03 | 0.09 |
| Serum bilirubin | 1.42 | 1.03–1.97 | 0.03 | |||
| ICU admission | 3.15 | 1.51–6.59 | 0.002 | |||
| Nephrotoxics drugs | 2.66 | 1.16–6.06 | 0.02 | |||
| Iodinated contrast media | 3.18 | 1.39–7.28 | 0.006 | |||
| Baseline SCr | 4.79 | 1.26–18.18 | 0.02 | |||
| SCr at admission | 3.91 | 1.34–11.37 | 0.01 | 6.20 | 1.57–20.50 | 0.009 |
| SCr at start CMS | 4.04 | 1.25–13.02 | 0.01 | |||
| Sepsis severity | ||||||
| Sepsis–severe sepsis | 5.87 | 1.49–23.05 | 0.01 | 8.07 | 2.46–26.41 | 0.001 |
| Sepsis–septic shock | 52.38 | 6.48–423.34 | 0.02 | 42.93 | 13.39–78.18 | <0.001 |
| Cumulative doses of CMS | 1.00 | 0.99–1.01 | 0.94 | |||
| Duration of CMS treatment | 1.01 | 0.98–1.04 | 0.34 | |||
Model performance: area under ROC curve=0.80 (0.73–0.88); Hosmer–Lemeshow χ2=0.77.
APACHE: Acute Physiology and Chronic Health Evaluation; CI: confidence interval; CMS: colistimethate sodium; ICU: intensive care unit; OR: odds ratio; ROC: receiver operating characteristic; SCr: serum creatinine level; SOFA: Sequential Organ Failure Assessment.
Forty-two patients (33%) died during the in-hospital follow-up. The incidence of death was significantly higher in patients that developed AKI (44%; 27/61), as compared to those who did not (23%; 15/65, P=0.032).
Evolution of the renal function at the 6-month follow-upWe evaluated the changes of the RF in the 84 surviving patients (66%) after hospital discharge, along with the prognosis factors.
In the univariate linear regression model, the results for the RF at the 6-month follow-up were significantly correlated with age (P=0.003), baseline eGFR (P=0.0001), eGFR at the time of CMS therapy initiation (P=0.01) and eGFR at the time of discharge (P<0.001). These variables were included in the multivariate analysis.
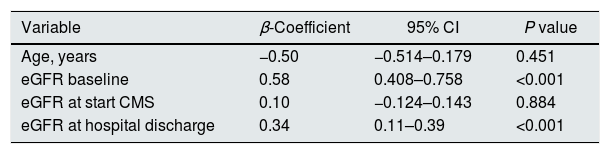
Length of CMS treatment, cumulative doses of CMS and the need of ICU admission were not found to be relevant. With a backward strategy, we found finally that, the baseline eGFR (0.58; 95% CI 0.40–0.75; P<0.001) and eGFR at discharge (0.34; 95% CI 0.11–0.39; P<0.001) emerged as the main predictors of eGFR at the six months of follow-up, with positive correlation (Table 3).
Final multiple linear regression model to predict GFR at six months of follow-up.
| Variable | β-Coefficient | 95% CI | P value |
|---|---|---|---|
| Age, years | −0.50 | −0.514–0.179 | 0.451 |
| eGFR baseline | 0.58 | 0.408–0.758 | <0.001 |
| eGFR at start CMS | 0.10 | −0.124–0.143 | 0.884 |
| eGFR at hospital discharge | 0.34 | 0.11–0.39 | <0.001 |
R2C=0.687.
CMS: colistimethate sodium; eGFR: estimated glomerular filtration rate; SOFA: Sequential Organ Failure Assessment.
Twelve patients died during follow-up, 4 of then developed AKI during hospital stay. The cause of death was severe infectious (ventriculitis, encephalitis and urinary sepsis) and terminal lung cancer.
Of the 8 patients who did not developed AKI during hospital stay, the causes of death were severe infection (4) and terminal neoplasms (4).
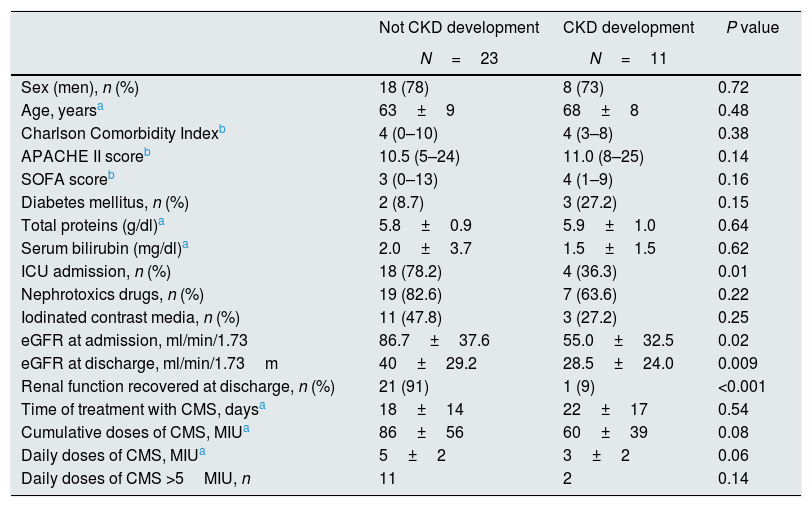
Risk factors for the development of chronic kidney disease after acute kidney injuryAt the 6-month follow-up, 56% of the patients (34/61) who developed AKI during their hospitalization survived. At this time, 11 patients (32%) had CKD. The main characteristics of patients according to the development of CKD are displayed in Table 4.
CKD development at 6 months of follow-up in patients with AKI.
| Not CKD development | CKD development | P value | |
|---|---|---|---|
| N=23 | N=11 | ||
| Sex (men), n (%) | 18 (78) | 8 (73) | 0.72 |
| Age, yearsa | 63±9 | 68±8 | 0.48 |
| Charlson Comorbidity Indexb | 4 (0–10) | 4 (3–8) | 0.38 |
| APACHE II scoreb | 10.5 (5–24) | 11.0 (8–25) | 0.14 |
| SOFA scoreb | 3 (0–13) | 4 (1–9) | 0.16 |
| Diabetes mellitus, n (%) | 2 (8.7) | 3 (27.2) | 0.15 |
| Total proteins (g/dl)a | 5.8±0.9 | 5.9±1.0 | 0.64 |
| Serum bilirubin (mg/dl)a | 2.0±3.7 | 1.5±1.5 | 0.62 |
| ICU admission, n (%) | 18 (78.2) | 4 (36.3) | 0.01 |
| Nephrotoxics drugs, n (%) | 19 (82.6) | 7 (63.6) | 0.22 |
| Iodinated contrast media, n (%) | 11 (47.8) | 3 (27.2) | 0.25 |
| eGFR at admission, ml/min/1.73 | 86.7±37.6 | 55.0±32.5 | 0.02 |
| eGFR at discharge, ml/min/1.73m | 40±29.2 | 28.5±24.0 | 0.009 |
| Renal function recovered at discharge, n (%) | 21 (91) | 1 (9) | <0.001 |
| Time of treatment with CMS, daysa | 18±14 | 22±17 | 0.54 |
| Cumulative doses of CMS, MIUa | 86±56 | 60±39 | 0.08 |
| Daily doses of CMS, MIUa | 5±2 | 3±2 | 0.06 |
| Daily doses of CMS >5MIU, n | 11 | 2 | 0.14 |
APACHE: Acute Physiology and Chronic Health Evaluation; CKD: chronic kidney disease; CMS: colistimethate sodium; ICU: intensive critical care; MIU: million international unit; SOFA: Sequential Organ Failure Assessment.
Among the 23 patients that did not develop CKD, when analyzed at sixth months of follow-up, 21 (91%) had recovered totally their RF at discharge.
DiscussionThe results of this study show that CMS therapy is associated with an increased risk of AKI, being RF at admission and the severity of sepsis, the most important predictors of this complication.
In this study, 48% of the patients treated with intravenous CMS developed AKI as defined by KDIGO criteria.16 The majority of them were of mild or moderate intensity and only 29.5% reached stage 3. These results are in line with those reported by Miano et al.,18 using the same criteria used in this work for defining AKI in 150 patients also treated with CMS. Incidence of AKI was similar to ours (51%). The distribution of the severity of their episodes of AKI was according with that observed in this study. However, in recently published series a great variability can be observed, from 13% to 62% in the incidence of AKI in patients treated with colistin.19–33 The definitions of AKI used in these studies did not justify differences, given that the variation in its frequency (26–51%) in the studies that use the KDIGO criteria20,26,31,32 is similar (35–62%) to those who use the RIFLE classification21,27,29 with the exception of the 13% reported by Ghafur et al.30 Even though the time of detection of AKI varies among studies, a multicenter, retrospective study with a control group observed that 20% of the AKI associated with CMS had been detected on the third day of treatment.18 In our cohort the detection of AKI was after 13 days of treatment. In any case, the incidence of AKI in a group of patients treated with CMS increased with the follow-up time of the cases included.18,22
The analysis of the potential effect of CMS dose in the development of AKI has different perspectives. In our study, neither the daily dose of CMS – even when stratified to a dose higher than 5MIU/day – nor the cumulative CMS dose, or the length of its administration were associated with the development of AKI; however, our data should be interpreted carefully, due to the absence of CMS plasma levels to adjust the dose and dose interval. Sorli et al.,22 using RIFLE criteria to define AKI, demonstrated that nephrotoxicity associated with the use of CMS was correlated with the trough serum levels of CMS (measured before the administration of intravenous CMS) on day 7, and when the CMS therapy finished. In line with our results, the authors did not find any differences with the length of therapy nor the cumulative CMS dose,22 suggesting the potential clinical utility of monitoring serum CMS levels to prevent AKI in these patients.
The results of this study do not match with a previous study reporting that length of treatment and high-dose CMS (>9MIU/day) are risk factors for AKI.34 Rocco et al.,21 in agreement with our observations, reported that CMS therapy at high dose for more than 7 days does not appear to be a risk factor for AKI. Even though in our study the loading dose of CMS administered was not analyzed, its possible effect in the genesis of AKI is controverted, studies having been published in favour of34 and against35 this hypothesis.
In multivariate analyses, the severity of sepsis and SCr at admission in patients with MDRGN, emerged as independent predictors of AKI (Table 2).
The information above should be interpreted within the context of patients undergoing CMS treatment. In fact many of them are complex; patients suffer from infections with MDRGN bacteria and present several complications associated with high morbidity and mortality and long hospital stays. Therefore, we could not state that there is an isolated effect from CMS on the development of AKI. Rather, there are multiple factors that may contribute to AKI occurrence in this clinical setting such as hypovolemic or exposure to nephrotoxic therapies.
Indeed, some experimental studies23,36 have found an accumulation of the drug in the renal cortex that reinforce the inherent deleterious effect of this drug.37
AKI is a known risk factor for the development of CKD.7 However, limited data exists regarding survival and long-term renal outcomes in patients treated with CMS.
The results of this study show that RF outcome at the 6-month follow-up is independently correlated with both, eGFR at baseline and eGFR at discharge. Interestingly, neither the length of CMS treatment, the cumulative CMS dose and the ICU admission had any effect on the RF at the 6-month follow-up in our model.
In agreement with us, Ponte et al.,38 also observed, in a cohort of 157 acute tubular necrosis patients, that renal function at discharge was an independent predictor for renal function in the long term outcome.
In a recent observational study, Meraz-Muñoz et al.,8 compared the evolution of RF at 6 months of discharge of 29 patients, with a prior eGFR higher than 60ml/min/1.73m2, who had suffered AKI associated with CMS, with the evolution of 58 patients that had suffered bacterial infections and had been treated with other antibiotics but not colistin and had also suffered AKI. Both groups were balanced regarding age, sex, baseline eGFR, place of treatment, diabetes mellitus and concomitant use of vancomycin. Twenty patients treated with colistin (75%) developed CKD while only 16 people (22%) of the control group did. The independent risk factors were use of colistin and age. One limitation of this manuscript was the authors did routinely not measure serum CMS levels.
In cohort, 34 patients that developed AKI during hospital stay survived: twenty three of them (68%) recovered RF at 6 months after discharge, while 11 (32%) developed CKD. Unfortunately, due to the small number of events, we could not carry out a multivariate analysis. However, in the univariate analysis we did not observe either that length of treatment, cumulative dose, nor daily adjusted to 5million UI/day of CMS had any influence in the progression of CKD. The majority of patients who had complete recovery RF at six months had already achieved this outcome at discharge. These findings point out the importance of RF at the moment of discharge as a predictor or renal outcomes at six months of follow-up.
This study has several potential limitations. First, the determination of serum creatinine as well as the eGFR at the time of hospital admission is not ideally suited to measure kidney function in an unstable metabolic setting as it is in AKI.
Second, we did not use CMS loading dose strategy because the protocol study was designed before this practice was recommended in the literature.
Third, a major weakness of this study was to not measure and monitored CMS levels, which might have helped to evaluate the correct dose of CMS and avoid the risk of AKI as described by Sorli et al.22 This drug must be quantified for determining an appropriate dose in order to mitigate resistance and decrease its toxicity.
Although this study has drawbacks and limitations and only included a relatively small number of patients, it contributes to the understanding the effects of AKI in septic patients treated with CMS and the factors associated with the long-term outcomes of RF. Renal function in patients with sepsis treated with CMS should be screened aggressively to identify an early development of AKI allowing an adequate care planning. Further research is needed to allow us to understand factors involved in and the likelihood of renal recovery. The inclusion of biomarkers should contribute to an early diagnosis of AKI and to prevent the risk of CMS-related nephrotoxicity.
In conclusion, the present study shows that the risk of AKI in patients treated with CMS is high, although most of the cases are of mild or moderate severity. The main factors associated with the development of AKI were renal function at the time of hospital admission and the severity of sepsis. The majority of patients recovered their baseline RF at six months after discharge. This study provides that, a decreased eGFR at the time of hospital discharge could help to predict the renal outcomes in those who developed CKD.
Further prospective studies are warranted to better elucidate the main underlying mechanisms for AKI in patients treated with CMS, how to manage it and prevent its appearance.
Conflict of interestsThe authors declare no conflicts of interest related to the publication of this article.
Vicente Pintado has served as a speaker of educational activities funded by GES (Generic Spanish Laboratory S.A.).