Dear Editor:
Anti-glomerular basement membrane (GBM) antibodies are sometimes detected in patients with sera that contain antineutrophil cytoplasmic antibodies (ANCAs), especially in those with specificity for myeloperoxidase (MPO)1-5. Double positive patients may have a clinical course and response to treatment more typical of vasculitis than of anti-GBM disease, and renal function recovery may be more likely if ANCAs are present1. Recent observations3-5 however, have failed to detect the differences described in early reports.
Here, we present a case of a 62 years-old Caucasian female with rhinosinusitis and asthma diagnosed 2 years before was admitted at our Department with renal failure requiring dialysis. She had anorexia, weight loss (15% of body weight), and weakness over the last 7 months. One month before the hospitalization she developed fever, persistent cough, dyspnea, myalgias, arthralgias, numbness and weakness of lower limbs. Physical examination revealed pale skin, blood pressure of 135/78 mmHg, heart rate of 68 beats per minute, respiratory rate of 18 cycles per minute, body temperature of 36.3 °C, oliguria (350 ml/day). Cardiac, pulmonary and abdominal examination did not reveal any changes. There was no ocular inflammation, joint tenderness or effusion, and rash. Neither edema nor tenderness of lower limbs, nor peripheral lymphadenopathies was present. Neurologic examination disclosed asymmetrical motor and sensory compromise of lower limbs.
Laboratory disclosed microcytic hypochromic anemia (hemoglobin, 4.8 g/dl; mean globular volume, 76.9 fl; mean globular hemoglobin, 24.1 pg), leucocytosis (14.460/mm3), eosinophilia (2.300/mm3), thrombocytosis (880.000/mm3), elevation of erythrocyte sedimentation rate (142 mm/hour) and of C-reactive protein (20 mg/dl), renal failure (uremia, 199 mg/dl; creatinemia, 6.1 mg/dl), and hiperkalemia (8.2 mEq/l). Urinalysis showed proteinuria of 100 mg/dl and 200 erythrocytes/microliter. Serum protein electrophoresis revealed IgG/K monoclonal gammopathy. Hepatic function tests, lactate dehydrogenase, calcemia, and phosphatemia were on the normal range.
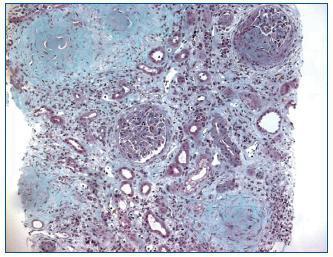
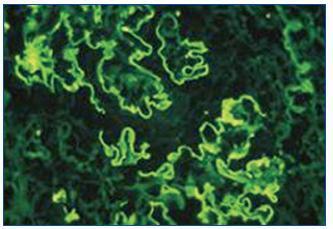
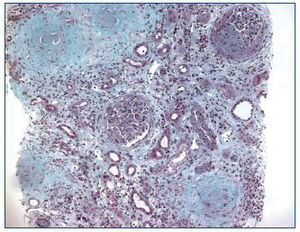
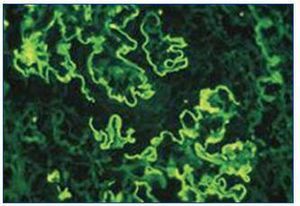
Renal ultrasound revealed normal sized kidneys, cortical hyperechogenicity, normal parenquimatous differentiation, and no hydronephrosis. Computed tomography (CT) of the face disclosed mucous thickening of the frontal, ethmoid, sphenoid and maxillary sinuses lining, and CT of the chest revealed ground-glass opacities widely spread across both lungs. Lower limbs electromiogram revealed severe multiple mononeuritis. Bone marrow biopsy disclosed eosinophilic hypercellularity and no morphologic abnormalities. A renal biopsy was performed and showed CGN with linear deposition of IgG along the glomerular capillaries (Figure 1 and figure 2). Serology for lupus (antinuclear, anti-double strand deoxyribonucleic acid, anti-Smith, extractable nuclear and anti-ribonucleoprotein antibodies) was negative. Serum C3, and C4 were on the normal range. Serology for human immunodeficiency virus types 1 and 2, hepatitis B, hepatitis C was also negative. Indirect immunofluorescence assay detected perinuclear ANCA (p-ANCA) (90 U/ml) and enzyme-linked immunosorbent assay (ELISA) revealed MPO specificity. Anti-GBM antibodies in serum (169 U/ml) were detected by direct ELISA. According to these, the diagnosis of CGN with double-positivity for anti-GBM antibodies and MPO-ANCA was established. She underwent intermittent hemodialysis, and immunosupressive therapy with metilprednisolone (15 mg/kg/day, 3 days, IV) followed by oral prednisone (1 mg/kg/day), cyclophosphamide (750 mg/m2, monthly, IV), and plasma exchange with daily exchange of one volume of plasma for 5% human albumin for 14 days was initiated. She also received red blood cell transfusions. Two weeks later, the patient was asymptomatic, recovered diuresis, and improved renal function. At hospital discharge (day 38), uremia and creatinemia were 148 mg/dl and 2.5 mg/dl, respectively, and hemoglobin was 10.9 g/dl. She had no clinical or laboratorial evidence of disease relapse, and she remains out of dialysis.
This case illustrates several interesting points. The patient presented with symptoms and signs in other organs suggesting systemic vasculitis. Serologic tests revealed coexistence of anti-GBM antibodies and MPO-ANCA, and histology showed CGN with linear deposition of IgG along the glomerular capillaries. Although the patient presented with renal failure requiring dialysis, there was renal function recovery after immunosupression with plasma exchange, without evidence of disease relapse.
In a substantial proportion of patients with CGN double-positivity for anti-GBM antibodies and ANCAs (mostly MPO-ANCA) is detected1-5. Double-positive patients may have a clinical course and response to treatment more typical of vasculitis than of anti-GBM disease, and have possibly developed anti-GBM antibodies secondary to vasculitic glomerular damage. Some patients have symptoms and signs in other organs suggesting systemic vasculitis, and renal function recovery may be more likely if ANCAs are present1. Recent observations3-5 however, have failed to detect the differences described earlier. Rutgers et al.4 reviewed 46 MPO-ANCA-positive, 10 double-positive and 13 anti-GBM-positive patients with CGN. Creatinemia was lower in ANCA-positive patients compared to double-positive or anti-GBM-positive patients (5.0, 10.3, 9.6 mg/dl, respectively; P = 0.01), and renal survival was different among the 3 groups (65%, 10%, and 15% of dialysis at 1 year, respectively; P = 0.04). Levy et al.3 analyzed 27 patients with CGN and double-positivity for anti-GBM antibodies and ANCAs (mostly MPO-ANCA), and described patient and renal survival rates of 52% and 26%, respectively, at one year. Sixty-eight percent of patients were dialysis-dependent at presentation, and none of these recovered renal function, despite immunosuppression with or without plasma exchange.
Although patients with CGN and double-positivity for anti-GBM antibodies and ANCAs may have a poor prognosis when presenting with severe disease, behaving more like anti-GBM disease than vasculitis, and recovery from severe renal failure may be rare, this case highlights that immunosupressive therapy with plasma exchange can improve patient and renal outcome in such patients.
Figure 1. Kidney biopsy showing crescentic glomerulonephritis (Masson trichrome, x100).
Figure 2. Immunofluorescence microscopy showing linear deposition of IgG along the glomerular capillaries (x400).