Background: Macroscopic haematuria secondary to renal cyst rupture is a frequent complication in autosomal dominant polycystic kidney disease (ADPKD). Sickle-cell disease is an autosomal recessive haemoglobinopathy that involves a qualitative anomaly of haemoglobin due to substitution of valine for the glutamic acid in the sixth position of 3-globin gene on the short arm of chromosome 11. For the full disease to be manifested, this mutation must be present on both inherited alleles. The severity of the disease is proportional to the quantity of haemoglobin S (Hb S) in the red cells; sickle-cell trait (Hb S <50%) and homozygous sickle-cell disease (Hb S >75%). In sickle-cell disease, the abnormal Hb S loses its rheological characteristics and is responsible of the various systemic manifestations including those of the kidney, such as macroscopic haematuria secondary to papilar necrosis. Despite the generally benign nature of the sickle-cell trait, several potentially serious complications have been described. Metabolic or environmental changes such as hypoxia, acidosis, dehydration, hyperosmolality or hyperthermia may transform silent sickle-cell trait into a syndrome resembling sickle-cell disease with vaso-occlusive crisis due to an accumulation of low deformable red blood cells in the microcirculation originating haematuria from papilar necrosis. On the other hand, it has been demonstrated an earlier onset of end-stage renal disease (ESRD), in blacks with ADPKD and sickle-cell trait when compared with blacks with ADPKD without the trait. Patients and methods: We studied 2 african-american families (4 patients) which presented with both ADPKD and sickle-cell trait (Hb S <50%). The diagnosis of sickle-cell trait was confirmed by haemoglobin electrophoresis. The renal volume was measured by magnetic resonance imaging (MRI). Results: The proband subject in family 1 presented frequent haematuria episodes, associated to increase of renal volume, developed very early ESRD and was dialyzed at the age of 39 years. The other 3 patients in family 2 presented different degree of renal function. Conclusions: The presence of sickle haemoglobin should be determined in african-american and west-african patients with ADPKD because it is an important prognostic factor. Co-herence of sickle-cell trait may have influence on ADPKD evolution to ESRD and other complications, such as cystic haemorrhages. MRI can identify intracystic haemorrhage and permit renal volume measure.
Antecedentes: La hematuria macroscópica derivada de la rotura de quistes renales es una manifestación habitual en la poliquistosis renal autosómica dominante (PQRAD). La enfermedad por células falciformes es una anomalía de la hemoglobina, que se hereda con carácter autosómico recesivo, consistente en la sustitución de la valina por el ácido glutámico en la posición 6 del gen de la 3-globina en el brazo corto del cromosoma 11. La gravedad de la enfermedad es proporcional a la cantidad de hemoglobina S (Hb S) en los hematíes: los heterocigotos con hemoglobina con rasgo falciforme (Hb S <50%) y los homocigotos con enfermedad por células falciformes (Hb S >75%). La presencia de hemoglobina con rasgo falciforme (Hb AS) se acompaña de manifestaciones renales, especialmente hematuria, y la necrosis papilar es la causa más frecuente de hematuria macroscópica en los pacientes heterocigotos portadores de esta hemoglobinopatía. La asociación de estas dos enfermedades hereditarias, PQRAD y hemoglobina con rasgo falciforme, se ha comunicado raramente. Se ha sugerido que los pacientes con PQRAD y hemoglobina con rasgo falciforme podían desarrollar precozmente insuficiencia renal crónica (IRC). Recientemente, se ha comunicado que la hemoglobina con rasgo falciforme es un factor de riesgo predisponente para el desarrollo de enfermedad renal crónica en afroamericanos. Pacientes y métodos: Se estudiaron 2 familias de origen afroamericano (4 pacientes) que co-heredaron la PQRAD y la hemoglobina con rasgo falciforme (heterocigotos). El diagnóstico de hemoglobina falciforme (Hb S) se realizó por electroforesis de la hemoglobina. El volumen renal se midió mediante resonancia magnética (RM). Resultados: La paciente índice, perteneciente a una de las familias, presentó episodios de hematuria macroscópica recidivantes, asociados con incremento del volumen renal y desarrollo precoz de IRC avanzada, precisando tratamiento con diálisis a los 39 años de edad. Las 3 pacientes pertenecientes a la otra familia, de tres generaciones diferentes, presentaron distintos grados de función renal. Conclusiones: En los pacientes afroamericanos y del África subsahariana con PQRAD, debe determinarse la presencia de hemoglobina falciforme, porque ésta puede ser un importante factor pronóstico. La co-herencia de PQRAD y hemoglobina con rasgo falciforme puede influir en la evolución hacia la IRC y en el desarrollo de complicaciones, como el sangrado quístico. La imagen de RM es una herramienta de utilidad para identificar las hemorragias quísticas y para medir el volumen renal.
INTRODUCTION
Polycystic kidney disease is an inherited, autosomal dominant disease caused by mutations in two genes, PKD1 (the short arm of chromosome 16) and PKD2 (the long arm of chromosome 4). It is characterised by the presence of renal cysts that gradually increase in number and size, leading to end-stage chronic renal failure at an average age of 50-60 years. In autosomal dominant polycystic kidney disease (ADPKD), macroscopic haematuria resulting from the rupture of renal cysts is a common manifestation.1
Drepanocytosis, or sickle cell disease, is inherited as an autosomal recessive haemoglobin abnormality caused by the substitution of valine for glutamic acid at position 6 in the β-globin gene on the short arm of chromosome 11. The severity of the disease is proportional to the amount of haemoglobin S (HbS) in red blood cells: sickle cell trait (HbS<50%) and homozygous sickle cell disease (HbS>75%). In sickle cell disease, abnormal haemoglobin S loses its rheological properties and is responsible for several systemic manifestations, including those of the kidney, such as papillary infarcts due to vascular lesions. In sickle cell disease, chronic renal failure (CRF) may be a complication in up to 4%-20% of cases. The presence of sickle cell trait (HbAS) may also be associated with renal manifestations, especially haematuria. Papillary necrosis is the most common cause of macroscopic haematuria in heterozygous patients with sickle cell trait.2-6
The association of these two hereditary diseases, ADPKD and sickle cell trait, has been rarely reported in the literature. In the 1990s, it was reported that African American patients with ADPKD, who also had sickle cell trait, could develop ESCRF earlier.7,8 Since then, there have been no references to families or series with this genetic association, despite the relative frequency of both hereditary diseases. Four patients belonging to 2 African American families, with co-inheritance of ADPKD and sickle cell trait (heterozygous) are described. In one case, the patient developed ESCRF at 39 years of age after numerous recurrent episodes of macroscopic haematuria. The other 3 patients had varying degrees of renal function.
PATIENTS AND METHODS
The diagnosis of ADPKD was made from the family history and related imaging tests that included ultrasound, magnetic resonance imaging (MRI) and in some cases, computed tomography (CT). Although there were no DNA genetic studies, the ADPKD was in all probability PKD1 (chromosome 16), taking into account the form of presentation, clinical features and time of diagnosis in these families. The first family consisted of two generations and the second of three. The diagnosis of sickle cell trait (HbS) was performed by electrophoresis of haemoglobin in acid and alkaline media. The total renal volume was determined by non-enhanced MRI in T1 and T2 weighted sequences, and by manual segmentation technique, adding the volume of both kidneys. In all patients with recurrent haematuria, the presence of renal medullary carcinoma was ruled out. Figures 1 and 2 show both family trees. Figures 3, 4 and 5 show representative images of the polycystic kidneys. Tables 1 and 2 summarise the clinical and developmental data of the patients.
Index Case (Case 1)
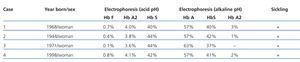
An African American woman born in 1968 (a native of Santo Domingo) who was diagnosed with ADPKD at 35 years old after renal ultrasound, which was performed due to an episode of renal colic with passage of several blood clots. Her family history showed that her father ha been diagnosed with ADPKD, and had undergone haemodialysis treatment since 55 years old. A younger sister was also diagnosed with ADPKD, although her degree of renal function was unknown. Her mother, the younger sister and the patient herself were carriers of sickle cell trait (HbAS). She was studying in Germany in April 2005 when she began with right flank pain and dark haematuria with clots. She had to be hospitalised and was diagnosed with a complicated renal cyst. At that time she had a SCr of 2mg/dl and Hb of 5.2g/dl, transfusion of 4 units of packed red blood cells was required. A week later, she was re-admitted for recurrent pain in the right flank, requiring strong analgesia. Following the completion of cystoscopy, a bladder mass compatible with clots was discovered which required 2 more transfusions. She received antibiotics and symptomatic treatment, and her anaemia improved to Hb 13.6g/dl and SCr 1.9mg/dl. An analytical control in October 2006 revealed SCr 2.4mg/dl and Cr clearance of 29ml/min. By MRI, the volume of the kidneys was RK 1392ml and LK 1485ml (total renal volume of 2877ml), and several cysts with signs of intracystic bleeding. Haemoglobin electrophoresis showed the following distribution: HbS 40%, HbA 57% and HbA2 3% (Table 1). Between 2006 and 2008 she had several episodes of recurrent haematuria with clots, accompanied by anaemia, which required multiple transfusions. In June 2008, her analytical results were SCr 4.4mg/dl and SCr clearance 15ml/min. By MRI, the volume of the kidneys was RK 2748ml and LK 2076ml (total renal volume of 4824ml). After repeated episodes of haematuria (some spontaneous and one after an accidental fall) and anaemia not responding to medical treatment, including tranexamic acid, an embolisation was proposed, which was not accepted by the patient. In September 2008 a left nephrectomy was performed. Haemodialysis via a permanent jugular catheter was then required. Attempts (on two occasions) to conduct an arteriovenous fistula for haemodialysis were unsuccessful due to thrombosis. After two years on haemodialysis and having suffered persistent haematuria, an embolisation and right nephrectomy had to be performed in September 2010. Neither of the two surgical samples from the nephrectomies showed changes consistent with renal medullary carcinoma.
DISCUSSION
In ADPKD, macroscopic haematuria resulting from the rupture of renal cysts is a common manifestation. Thus, haematuria is a symptom in 35% of cases, and microscopic or macroscopic haematuria in 50%.1 The risk of haematuria appears to be associated with the presence of hypertension and with an increase in the size of the cysts. Although most patients report trauma and violent exercise as possible precipitating causes, no association has been unequivocally demonstrated. Currently, with the widespread use of imaging techniques, and specifically MRI, intracystic bleeding can be observed which had previously gone unnoticed in many cases. These facts are very important, as it is known that ADPKD patients who have frequent episodes of haematuria or evidence of intracystic haemorrhage have a more rapid progression to CRF. Moreover, the presence of sickle cell trait (HbAS) is characterised by renal manifestations, especially haematuria, with papillary necrosis being the most common cause of macroscopic haematuria in heterozygous carriers of this haemoglobinopathy.9-12 It is therefore not surprising that the co-inheritance of both genetic diseases causes a synergy in terms of the occurrence of these bleeding complications and in the development of CRF.
In family 1, one of the autosomal dominant diseases, ADPKD, was transmitted in the male line while the maternal line carried the other recessive, sickle cell trait (Fig. 1). In this family, the index case was a woman with two genetic diseases who developed rapidly progressing CRF and had to start haemodialysis at 39 years of age. In this patient, renal cysts formed and developed very early, and the association of sickle cell trait (HbAS) very probably favoured recurrent episodes of macroscopic haematuria, intracystic haemorrhage and early development of advanced CRF. It is worth noting that, in this case, the episodes of haematuria were sometimes preceded by an airplane ride lasting several hours (obviously in a position of relative hypoxia) or by minimal trauma. Moreover, sequential MRI images measuring renal volume showed a significant increase in a relatively short time (68% in 2 years). This was no doubt due to intracystic bleeding and the intrarenal haematomas detected in the later stages of the disease. They were confirmed by CT and finally pathophysiologically. This development contrasted with that of the father, who was not a sickle cell trait carrier and required haemodialysis treatment at 55 years old. In family 2, both diseases, ADPKD and sickle cell trait were inherited by the same (maternal) line (Fig. 2). In this family, ADPKD was diagnosed early in one of the members, at 11 years old (case 4). This patient and the mother (case 3) showed glomerular hyperfiltration. The grandmother (case 2), who had some episodes of macroscopic haematuria, developed CRF, with MR images of intracystic bleeding and a moderately elevated total renal volume. To our knowledge, this is the first study that has evaluated families with this genetic association in Europe.
Surprisingly, only two papers regarding this matter were found in the literature, both from the same group, which described the association of two genetic diseases, ADPKD and sickle cell trait in African Americans.7,8 In one of them, the African American patients with ADPKD and HbAS showed a faster progression towards ESCRF than those without the sickle cell trait.7 Moreover, there are few data in the literature examining the relationship between sickle cell trait and chronic kidney disease. Only recently, a US study reported a 50% higher prevalence of sickle cell trait in a population of African Americans with ESCRF, compared with that inferred from the haemoglobinopathy newborn screening programme, meaning that it may represent a risk factor for the development of chronic kidney disease.13,14
The mechanism by which sickle cell trait contributes to the progression of chronic kidney disease in ADPKD may be multifactorial.15 The repeated occurrence of localised areas of sickling and vein occlusion, leading to chronic microvascular damage, may cause chronic hypoxia and tubulointerstitial fibrosis. It is possible that sickle cell trait, coexisting with other conditions affecting the renal microvasculature, like ADPKD, could act synergistically to accelerate renal damage. It must be borne in mind that serum levels of angiogenic factors reveal a proangiogenic state in adults with sickle cell disease.16 It may be that the production of proangiogenic factor stimulants, present in both diseases and acting synergistically, are responsible for cystic bleeding and rapid progression to CRF in some cases of ADPKD.
The presence of sickle cell trait (HbAS) may also affect the course and care of patients with ESCRF, as it may be an independent risk factor for venous thromboembolism among African Americans.13 This predisposition towards thrombosis may affect the arteriovenous fistula failure and loss of vascular access, as occurred in our index case.
In conclusion, the existence of sickle cell trait should be determined in African American patients and those from West Africa with ADPKD, as its presence may be an important prognostic factor. The co-inheritance of sickle cell trait may influence complications in patients with ADPKD and the evolution of CRF. This is probably also applicable to other highly prevalent renal pathologies, such as hypertension and diabetes mellitus.
ACKNOWLEDGEMENTS
This study was partially funded by a grant from the Instituto de Salud Carlos III, the Spanish Ministry of Health an Innovation (EC08/00236) and the Research Activity Intensification Programme (Programa Intensificación Actividad Investigadora, Laín-Entralgo/CM) to RP.
Table 1. Clinical and haematological characteristics of the 4 patients with ADPKD and HbAS
Table 2. Kidney development data for the 4 patients with ADPKD and HbAS
Figure 1. Family tree for family 1
Figure 2. Family tree for family 2
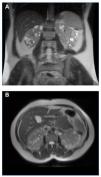
Figure 3. MRI of case 3 where the kidneys retain their shape, with multiple small cysts (TRV=603ml). A) Coronal view B) Axial view
Figure 4. MRI of Case 2 showing enlarged kidneys, with multiple large cysts, a number of which show signs of intracystic haemorrhage (TRV=1322ml). A) Coronal view B) Axial view
Figure 5. Axial view of CT in case 1 showing extremely enlarged kidneys, with multiple cysts with intracystic signs of haemorrhage-haematoma (TRV=2877ml).