CLINICAL GUIDE OF THE SPANISH SOCIETY OF NEPHROLOGY (SOCIEDAD ESPAÑOLA DE NEFROLOGIA) ON THE PREVENTION AND TREATMENT OF PERITONEAL INFECTION IN PERITONEAL DIALYSIS
More infoPeritoneal infections still represent a most feared complication of chronic Peritoneal Dialysis, due to their high incidence and relevant clinical consequences, including direct mortality, technique failure and a significant burden for the health system. The practices for prevention and treatment of this complication show a remarkable heterogeneity emerging, among other factors, from the complexity of the problem and from a paucity of quality evidence which could permit to respond clearly to many of the raised questions. The purpose of this document is to provide a complete and updated review of the main methods of diagnosis, prevention and treatment of these infections. The document has been elaborated taking as a reference the most recent guidelines of the International Society of Peritoneal Dialysis (2016). The diagnostic considerations are presented in a narrative style while, for prevention and therapy, we have used a systematic methodology (GRADE), which specifies the level of evidence and the strength of the proposed suggestions and recommendations and facilitates future updates of the document. The length of the document and the many suggestions and recommendations coming out of the review underline the large number and the complexity of the factors to be taken into consideration for an adequate approach to this complication of Peritoneal Dialysis.
Las infecciones peritoneales siguen constituyendo una complicación muy relevante de la diálisis peritoneal, por su incidencia todavía elevada y por sus importantes consecuencias clínicas, en términos de mortalidad, fracaso de la técnica y costes para el sistema sanitario. Las prácticas de prevención y tratamiento de esta complicación muestran una notable heterogeneidad derivada, entre otros factores, de la complejidad del problema y de la escasez de evidencia clínica que permitan responder de manera clara a muchas de las dudas planteadas. El propósito de este documento es proporcionar una revisión completa y actualizada de los métodos de diagnóstico, prevención y tratamiento de estas infecciones. El documento se ha elaborado tomando como referencia de partida la guía más reciente de la Sociedad Internacional de Diálisis Peritoneal (2016). Mientras que para el capítulo diagnóstico se ha adoptado una estructura más narrativa, el análisis de las medidas de prevención y tratamiento ha seguido una metodología sistemática (GRADE), que especifica el nivel de evidencia y la fuerza de las sugerencias y recomendaciones propuestas, y facilita actualizaciones futuras de la guía. La gran extensión y numerosas recomendaciones o sugerencias emanadas de la revisión ponen de manifiesto la complejidad y gran número de facetas a tener en cuenta para un adecuado abordaje de esta importante complicación de la diálisis peritoneal.
Despite a long history and the fact that peritoneal dialysis (PD) is fully established as a form of renal replacement therapy (RRT), clinical practice and the outcomes of the technique remain highly variable, as evidenced by the different national and international registries1–5. The causes of such variability are complex and heterogeneous throughout the world, and include social and sanitary factors such as the degree of socioeconomic development, the overall quality and accessibility of healthcare, different health policies, the assigned resources, differences between populations, and the role assigned to PD in the planning of RRT in each given country6. From a purely clinical perspective, the scarce robust evidence (multicenter and adequately weighted randomized clinical trials [RCTs]) in the field of PD is notorious, and has largely contributed to generate local practices based on observational and uncontrolled experiences.
In this scenario unfavorable to the success of RRT, many clinical practice guides on PD have been developed in recent decades at both national and international level. Ideally, only quality evidence would confer value to these guides, but the scarcity of such evidence makes them even more necessary for two main reasons:
- -
For less experienced professionals unable to resort to the conclusions drawn from well designed trials with solid results, the formal opinion of adequately constituted expert committees is of great help.
- -
It is strongly advisable to join and consolidate common criteria and practices, thus allowing the results obtained to be evaluated adequately.
In general, clinical practice guides in PD focus on the selection of patients, treatment adequacy, prescription and the complications of the technique — with preferential attention to infections. The usual approach is to address concrete questions of practical interest7–16, as in the standards of the International Society of Peritoneal Dialysis (ISPD) or the Caring for Australasians with Renal Impairment (CARI), with a view to developing a general body of recommendations. Only some guides, including the Spanish guidelines of 200517–19, adopt a more global approach.
The prevention and management of peritoneal infection (PI) has been a preferential issue for the main PD guides, either as chapters of general guidelines17–19 or as specific documents. Both the ISPD (https://ispd.org/ispd-guidelines/) and CARI (http://www.cari.org.au/Dialysis/dialysis%20peritonitis/dialysis_peritonitis.html) have been particularly active in this respect. The ISPD document of 201614 remains the most up-to-date reference, with some subsequent modifications20.
Purpose and scope of the guideRationaleIn general, the PD working group of the Spanish Society of Nephrology (Sociedad Española de Nefrología [S.E.N.]) accepts the ISPD guides14 as reference document in this field. However, we consider that renewal of the current PD guides of the S.E.N. is advisable, adapting them to our setting and the current scenario. The main reasons for this revision are:
- 1)
The wish on the part of the S.E.N. to establish a proprietary body of recommendations for the greatest possible number of scenarios in Nephrology.
- 2)
The time elapsed since the introduction of the PD guides in 2005, which were developed on a narrative basis with a non-systematic review of the literature that is inconsistent with the current methods for addressing issues of this kind.
- 3)
The great majority of the recommendations of the ISPD guides of 2016 are fundamentally based on opinions rather than on evidences, and some of them have been controversial to one degree or other; it is therefore advisable to address them from our own specific setting.
- 4)
The ISPD guides of 2016 have an international projection, and part of their recommendations are conditioned by economical and sociosanitary factors that are not applicable to our setting.
- 5)
Data compilation for the ISPD guides of 2016 ended in the closing months of 2015. Although this was not long ago, a 2021 update may be of interest.
The objective of this Guide is to offer recommendations that are up to date and focused on our sociosanitary setting, for the prevention and treatment of PI in PD, taking as starting point and reference the recommendations of the ISPD clinical practice guides of 201614.
Target populationThis Guide is mainly addressed to:
- -
Members of the S.E.N. in general
- -
Spanish, Latin American and Spanish-speaking nephrologists in general
- -
Residents in training in Nephrology
- -
Nursing professionals dedicated to PD
Its training objectives are also aimed at:
- -
Physicians of other specialties with an interest in infections in general, and in infections related to PD in particular
- -
Professionals, organizations and people with an interest in the subject, including patients with kidney disease and their associations
The group in charge of developing the clinical practice guide on PD was created under the auspices of the Steering Committee of the S.E.N. and the Clinical Practice Guides and Consensus Documents Coordinating Group of the S.E.N. It was organized through an Expert Committee designed by these organisms and composed of 9 professionals of solid prestige in the field, with Miguel Pérez-Fontán as coordinator. This Committee established the program, timelines and development of the Guide, and assigned sub-committees for preparation of the different chapters. Adoption of the GRADE (Grading of Recommendations, Assessment, Development and Evaluation) method led the Society to contract an external company (InMusc®) for methodological support and protocolized training of the members of the Expert Committee in the mentioned methodology. A series of methodological and financial limitations were recognized from the early stages of the project. The decision was therefore made to fractionate the project, placing priority on those aspects considered to be of greatest interest for the readers of the Guide (selection of patients, assessment of the peritoneal membrane, prescription and adjustment, and PI).
Development of the Guide for the prevention and treatment of periotenal infections was structured into four stages:
- -
Basic drafting of the Guide was carried out by the three panelists cited at the start of the document, with the key collaboration of Dr. Carlos Quereda on the part of the Methodological Group of the S.E.N.
- -
In a second stage, the chapter was evaluated by the Group of Experts of the clinical practice guide on PD.
- -
In a third stage, the document underwent external assessment by a group of professionals, including nephrologists, specialists in infectious diseases and nursing staff dedicated to PD, with exploration also of the opinion of the main association of patients with kidney disease (Alcer).
- -
Lastly, the document was opened to the general scrutiny of the S.E.N. through its website, before closure of the guide.
The methodology used in developing this document was based on the general principles of the GRADE system, summarized in the corresponding document-guide of the S.E.N., and reproduced in other S.E.N. documents of the same nature21–23.
A particular and fundamental element in the development of the Guide on PI has been the existence of a relatively recent guide (2016) on the same subject, developed by an Expert Committee of the ISPD14. The Group of Experts of the S.E.N. acknowledges the strong validity of the mentioned guide, with the exceptions described in the chapter on the purposes of the present document. The decision therefore was made to adopt the ISPD document as a basis and reference for the present Guide, as will be evidenced by the continuous references to the ISPD publication made in the course of the Guide. Some relevant issues are dealt with by other ISPD guides and documents that we have also used as references11,24,25.
In summary, the following steps were followed:
1) Development and review of clinical questions. These questions focused on the fundamental and potentially controversial aspects (prevention and treatment). A descriptive format was adopted for the general issues and aspects related to the diagnosis of PI.
2) Literature search strategy. Once the experts had defined the questions of interest on which to subsequently base the recommendations, the following was done:
- a)
A thorough review was made of the reference ISPD guide14, identifying and analyzing only those articles affording particularly relevant evidence related to the defined questions.
- b)
A systematic literature search on the subject was carried out, including publications posterior to the mentioned ISPD guide (2015−2019).
- c)
Before release of the chapter, thorough (non-systematic) searches were made of publications up until March 2021, of potential interest for the guide.
- d)
Tables corresponding to the PICO question21–23,26 for each clinical question were developed in order to define the criteria of the literature strategy, based on the following aspects:
- -
P = Population studied (patients with stage 5d chronic kidney disease, and treated with PD)
- -
I = Intervention studied (PI prevention or treatment measures)
- -
C = Comparator (other measure or alternative treatment or placebo, or no treatment)
- -
O = Outcome variables, classified according to clinical importance (infection rates, cumulative risk, mortality rates or failure of the technique related to infection, etc.).
- -
Adequate methodological designs (systematic reviews — meta-analyses, randomized and controlled clinical trials, prospective or retrospective cohort observational studies, case-control studies) were contemplated in the search strategy. The literature search was carried out by two documentalists in collaboration with the medical committee, designing a search strategy in accordance with the explicit indications of the PICO question, and exploring the MEDLINE (PubMed), Cochrane Library and EMBASE databases. The entire process was supervised by experts in systematic reviews and clinical practice guides (InMusc®).
3) Selection of articles. The identified articles were analyzed independently by two reviewers in order to select those studies that met the clinical criteria referred to inclusion/exclusion, interventions and comparator groups, outcome variables, study methods and methodological quality. Differences between the reviewers were resolved by consensus in those articles in which agreement was initially lacking.
4) Estimation of the quality of the evidence. A structured summary was made of the results of the relevant studies addressing each clinical question. For each outcome variable we evaluated the quality of the evidence according to the standard criteria defined by the GRADE system21, which rates the quality of the evidence as HIGH (A), MODERATE (B), LOW (C) or VERY LOW (D).
Due consideration was made of the following factors capable of modifying confidence in the results: risk of bias, consistency between the results of the available studies, the availability of direct evidence, and precision of the estimators of effect. In the case of observational studies, we took the following into account: size of the effect, dose-response relationship, and the possible impact of confounding factors upon the results27,28.
Each clinical question is accompanied by a summary of the findings from the literature review, stated at the end of each question in a section called “Summary of the evidence”.
The systematic search yielded a total of 673 potential publications between October 2015 and September 2019, but only 52 publications fully met the established criteria. An additional 43 publications were identified on a non-systematic basis between October 2019 and March 2021. The articles finally selected per question and the analytical forms established according to the GRADE criteria can be consulted in the Annexes.
5) Editing structure: This review is characterized by a teaching orientation that made it necessary to develop descriptive or conceptual aspects (definitions, classifications, conventions, organizational aspects, diagnostic procedures etc.) that have been addressed through traditional narrative constructs.
Editing of the results generated in response to the questions raised with the GRADE method has been carried out based on the following narrative scheme, supported by figures and tables:
- -
Introduction. Where applicable, a brief definition is provided of the clinical problem and its context.
- -
Synthesis or summary of the evidence. This section includes the data and literature support extracted from the ISPD guide14 in response to the questions developed by the expert panelists, selecting the supporting studies and scoring the quality of the evidence of the articles based on the GRADE system23,27,28.
In addition to the evidence documented in the ISPD guide, we contributed “new evidence” generated by our protocol for the search, selection and ranking of articles addressing the defined questions, identifying those from the previous guide and the new publications obtained by our search, with a brief description of their quality and relevance. This process in turn was completed by manual searches of the PubMed (MEDLINE) and Cochrane Library databases and reference lists of the recently published useful articles.
- -
Evidence of the recommendation and definition of recommendations. The team defined the grade of recommendation referred to each question, rated as 1 (Strong = recommended); 2 (Weak = suggested); or Not applicable or Not rated21,22,29. This section explains the process leading to the recommendations based on the existing evidence and other clinical and sociological considerations.
As has been mentioned, the recommendations of the working group were evaluated on a blind basis by all members of the group of experts of the Guide, resolving any discrepancies through a two-round Delphi survey.
Abbreviations| GNB | Gramnegative bacteria |
| CARI | Caring for Australasians with Renal Impairment |
| PD | Peritoneal dialysis |
| APD | Automated peritoneal dialysis |
| CNS | Coagulase-negative staphylococcus |
| CKD | Chronic kidney disease |
| CRI | Catheter-related infection |
| PI | Peritoneal infection / peritonitis |
| ISPD | International Society of Peritoneal Dialysis |
| PCR | Polymerase chain reaction |
| S.E.N. | Spanish Society of Nephrology |
| RRT | Renal replacement therapy |
| Prevention | |
| 1 | Do structured preventive strategies, including Continuous Quality Improvement (CQI), reduce the incidence of peritonitis/PI? |
| 2 | How do the experience of the trainer and the training protocol influence the incidence of peritonitis/PI? |
| 3 | Are there measures related to insertion of the peritoneal catheter capable of reducing the ulterior risk of peritonitis/PI? |
| 4 | Does the type of care following implantation of the peritoneal catheter influence the risk of peritonitis/PI? |
| 5 | Does the diagnosis and treatment of Staphylococcus aureus carriers reduce the incidence of peritonitis/PI due to gram-positive microorganisms? |
| 6 | What measures are particularly important during peritoneal dialysis exchange in order to reduce the risk of peritonitis/PI? |
| 7 | Does the type of PD system influence the incidence of peritonitis/PI? |
| 8 | Does automated PD reduce the frequency of peritonitis/PI? |
| 9 | Does the use of dialysis solutions buffered with bicarbonate and low in glucose degradation products (GDPs) (“biocompatible” solutions) reduce the incidence of peritonitis/PI? |
| 10 | Does antibiotic prophylaxis reduce the risk of peritonitis/PI after accidental disconnection? |
| 11 | What peritonitis/PI preventive measures should be applied in patients on PD who are to undergo endoscopic gastrointestinal, gynecological o bacteremic procedures? |
| 12 | Does antifungal prophylaxis reduce the risk of fungal peritonitis/PI in patients on PD who are treated with broad-spectrum antibacterials or during long periods of time? |
| 13 | Does treatment with vitamin D reduce the risk of peritonitis/PI? |
| Treatment | |
| 1 | What is the most appropriate antibiotic or antibiotic association for the empirical treatment of PI? |
| 2 | What is the effect of peritoneal lavage and the addition of heparin upon the course of PI in PD? |
| 3 | What is the most appropriate antibiotic administration route for the treatment of PI? |
| 4 | Are there differences in results between intermittent and continuous treatments for PI? |
| 5 | Are there differences in terms of the most appropriate antibiotic administration regimen between patients treated with continuous ambulatory peritoneal dialysis (CAPD) and automated PD? |
| 6 | What is the required duration of treatment in PI? When should it be prolonged? |
| 7 | When is the administration of an antibiotic combination for the treatment of PI indicated? |
| 8 | What is the most appropriate treatment for PI due to different types of bacteria in PD? |
| 9 | What is the most appropriate treatment for fungal PI in PD? |
| 10 | Is withdrawal of the peritoneal catheter in mycobacterial PI in PD indicated? |
| 11 | What treatment is indicated in PI with an atypical course? |
| 12 | What treatment is indicated for PI associated to simultaneous infection of the peritoneal catheter tunnel/exit site caused by the same microorganism? |
| 13 | What is the indicated time interval between withdrawal of a peritoneal catheter due to PI and the implantation of a new catheter? Is withdrawal and simultaneous implantation feasible in this context? |
Peritoneal infections (PIs) are a very common complication of PD, and for decades have been the main obstacle facing development of the technique. Such infections remain a cause of concern in PD units, in view of their still undesirably high incidence and other effects:
- -
From the clinical perspective, PI increases patient morbidity and can result in discontinuation of this dialysis technique2,30–35, and even death5,36,37. Each PI episode is associated to an increased mortality risk over the following months38,39, and a succession of infections can result in membrane failure and/or peritoneal membrane sclerosis.
- -
From the economical perspective, the treatment and complications of PI generate an important added cost for healthcare systems.
- -
Lastly, fear of PI is one of the factors associated with rejection of PD on the part of some patients.
Achieving very low levels of PI in a peritoneal dialysis program is possible, provided the risk factors (Table D1) and characteristics of the patient are correctly evaluated (Table D2), and the corresponding prevention and treatment recommendations are applied. With regard to the latter, it must be underscored that prevention, early diagnosis and treatment of the infections are primary objectives in the current protocols for the training and monitoring of patients on PD11,24,40,41.
General risk factors for peritoneal infection.a
| Non-modifiable | Old ageFemale genderRaceDiabetesAbdominal disease (diverticulosis, inflammatory bowel disease, cholelithiasis)Immune suppressionPrevious renal transplantPrevious hemodialysisLittle or no residual renal functionLow socioeconomic status |
| Modifiable | SmokingDomestic petsDistant from Dialysis UnitNo predialysis education and trainingUnscheduled startNegative selection of PDHepatitis CObesityDepressionMalnutritionHypokalemiaLow vitamin D levelsTreatment with gastric acid secretion inhibitorsStaphylococcus aureus carrier statusInvasive medical procedures |
Factors related to the patient conditions that can favor a lower risk of peritoneal infection.
| Free and informed choice of the PD technique |
| Motivation for self-care |
| Learning capacity (the patient understands and retains) |
| Manual skill and strength |
| Good vision |
| Autonomy (not dependent on caregivers) |
| Good social and family environment |
| Availability of a clean zone for the exchanges |
| Availability of means for material storage |
Over the last three decades there has been a gradual decrease in the incidence of PI in PD42. However, only the centers in the Anzdata setting have maintained a marked decrease in this incidence in recent years – this being attributed to the systematic application of comprehensive and structured 43 prevention and continuous quality improvement strategies5,44–46.
The purported main reasons underlying this decrease in infection rate are reflected in Table D3. Some of them (for example, connectology improvements) have had an undeniable impact47–49, while the relevance of others, such as the introduction of solutions low in glucose degradation products (GDPs) and partially or totally buffered with bicarbonate (hereinafter referred to as “biocompatible” solutions) is more controversial50–52.
Main factors that may have contributed to the decrease in incidence of peritoneal infection.
| Improvements in patient selection criteria for PD |
| Identification and action upon modifiable risk factors |
| Systematization of the training protocols |
| Improvements in monitoring (home visits, retraining) |
| Advances in connectology |
| Biocompatible solutions |
| Management of Staphylococcus aureus carriers |
| Improvements in peritoneal catheter insertion techniques |
| Improvements in peritoneal catheter care |
| Prophylactic treatment in risk situations |
| Integral prevention strategies (continuous quality improvement) |
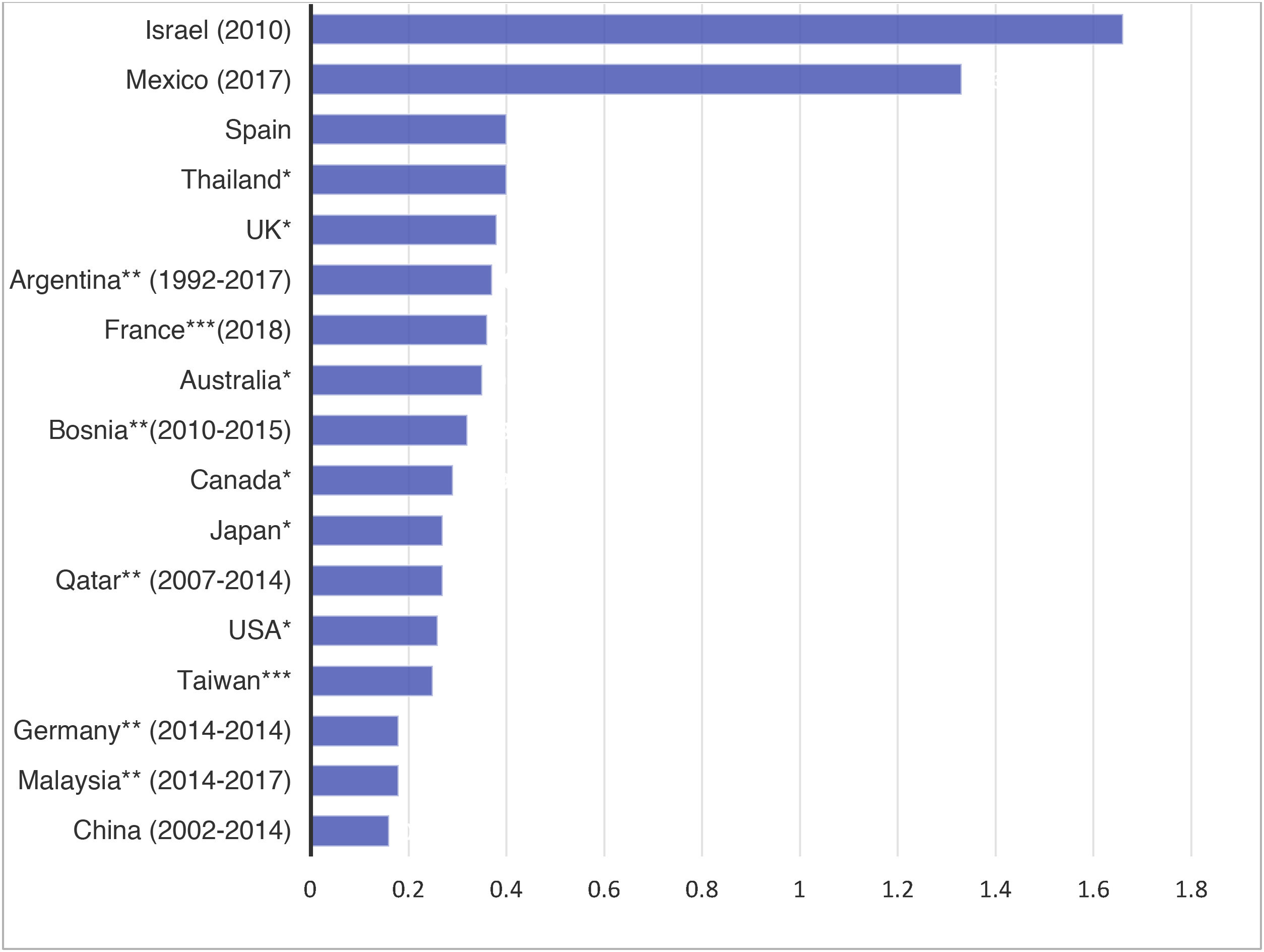
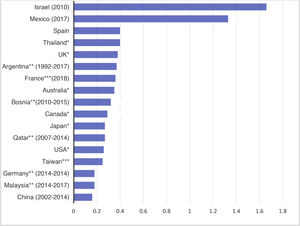
The international data are characterized by important variability of the mean incidences of PI (Fig. D1). This variability is also evidenced in the Spanish national registries. For instance, the USRDS registry reports rates as low as 0.06 episodes per patient and year in some centers, versus 0.77 in others. At present, it is not uncommon to find centers with infection rates of under 0.2 episodes per patient and year. Some of these differences may be explained by the different PD patient inclusion criteria used, or the different ways of quantifying the incidence of PI. The ISPD considers rates in excess of 0.5 episodes per patient and year to be inadequate14.
As mentioned above, important variability is also observed in Spain, though most centers present acceptable infection rates according to the current standards2.
Quantification of the incidenceIn view of the great variability of the epidemiology of PI, the ISPD advises each PD program to record its global PI rate at least once a year, along with the number of infection-free patients, the infection rate according to the causal agents, and the antimicrobial susceptibility of the most common pathogens.
In order to allow effective comparison among registries, it is necessary to quantify and monitor PI in the same way in all centers53. Different formulas or quantification methods have been described, though most experts recommend the expression of infection rates as episodes per patient and year. The parameters most commonly used for the control and registry of PI in the context of a PD program are described in Table D4.
Methods for quantifying the incidence of peritoneal infection.
| Parameter | Formula | Standard | Disadvantages |
|---|---|---|---|
| Incidence rate | Numerator: no. of episodes of peritonitis recordedDenominator: sum of risk exposure time of all patients (in years) | <0.5 | Does not reflect variability among patients and assumes homogeneous distribution of peritonitis in the evaluated time interval |
| Patient specific rate | Numerator: no. of episodes of peritonitis in a concrete patientDenominator: sum of risk exposure time of that patient (in years) | – | Not applicable if follow-up is very short or less than the studied time interval |
| Months between episodes | Numerator: sum of risk exposure time of all patients (in months)Denominator: no. of episodes of peritonitis recorded | >24 months | |
| Percentage of patients free of infection | Numerator: no. of patients who have suffered some peritonitis episode x 100Denominator: total patients in PD program exposed in the study period | >85% | |
| Infection rate per concrete microorganism | Numerator: no. of peritonitis episodes caused by the evaluated microorganism Denominator: sum of risk exposure time of all patients (in years) | Coagulase-negative staphylococci <0.03S. aureus <0.03 | |
| Percentage infection per concrete microorganism | Numerator: no. of peritonitis episodes caused by the evaluated microorganism x 100 Denominator: total no. of peritonitis episodes | Grampositive: 60−70%,Gramnegative: 10−30%Fungi: <5% | |
| Percentage infections with negative culture | Numerator: no. of infections with negative culture x 100Denominator: total no. of infections | Acceptable <15%Ideal <10% | |
| Percentage healing | Numerator: no. of healed episodes x 100Denominator: total no. of peritonitis episodes | >80% |
Other criteria also must be followed for standardization purposes:
- 1)
It is the dominant (albeit not unanimous) criterion of this Committee that the risk period should start to be counted from the start of patient training.
- 2)
The risk period should end at the time of kidney transplantation, permanent transfer to hemodialysis, or death.
- 3)
In the case of temporary transfer to hemodialysis, the period of treatment with this technique should not be counted.
- 4)
Repeated or recurrent infections, but not relapses, are to be counted as new episodes.
Peritoneal infection (PI) is defined as invasion of the peritoneal cavity (including the peritoneal membrane, adjacent tissues and the dialysate itself) by infectious agents, with the consequent inflammatory response, which usually causes the problem to become visible and leads to the diagnosis.
Microorganisms may be present in the peritoneal space without any evident clinical response. This situation in theory may correspond to noninvasive colonization, the clinical (but not bacteriological) remission phase of a treated infection, or to patient incapacity to develop an inflammatory response. Contamination of the sample is always a possibility in these cases.
On a standardized basis and as ratified by the most recent guides14, the diagnosis of PI in PD requires the presence of at least two of the following criteria:
- 1
The presence of clinical signs and/or symptoms of peritoneal inflammation, including abdominal pain, a positive rebound (Blumberg) test, nausea, vomiting, diarrhea or fever.
- 2
Turbid peritoneal fluid drainage, with the condition that turbidity (clouding) is attributable to an elevated leukocyte count in the dialysate, standardized to a minimum of 100 cells per mm3 and >50% of polymorphonuclear (PMN) cells. These limits must be individualized, however:
- -
In some cases, pain precedes the cellular response in the effluent by some hours.
- -
In the case of samples obtained after short dwells, the presence of > 50% of PMNs is already a sign of PI, even if the total leukocyte count does not exceed 100 cells per mm3.
- -
In some specific infections (especially those caused by mycobacteria)54 or in patients already treated with antibiotics at the time of diagnosis of the infection, the observed leukocyte response may be predominantly monocytic.
- -
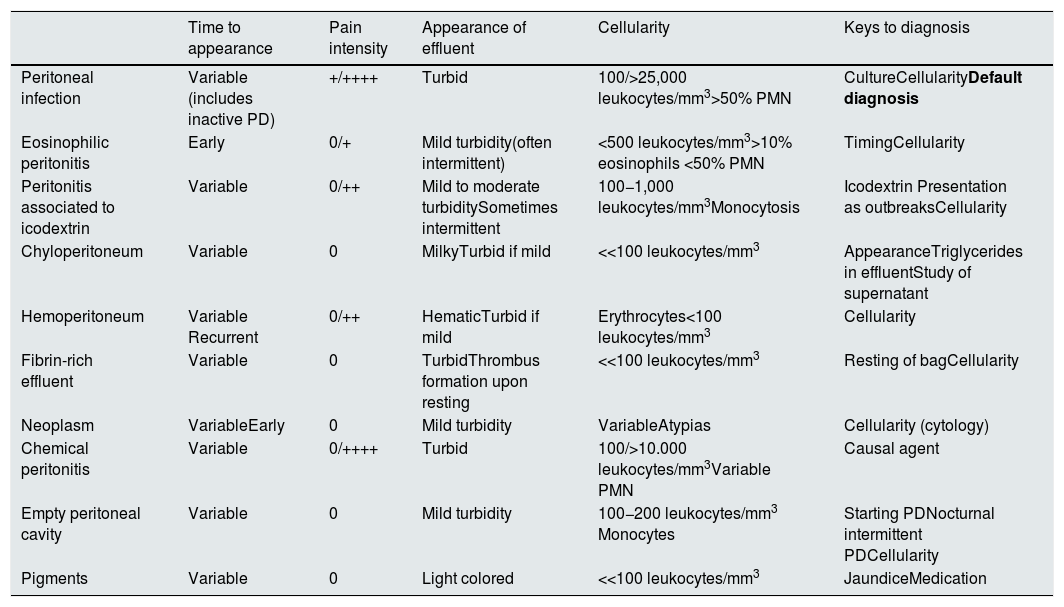
The study of peritoneal cellularity is of great help in the diagnosis of PI, and together with other clinical or biochemical characteristics facilitates the differential diagnosis in the presence of turbid peritoneal fluid drainage (Table D5).
- 3
Microbiological confirmation based on the visualization of microorganisms through direct Gram staining, their isolation in cultured dialysate samples, or their detection using other validated methods (e.g., PCR-based detection of microbial nucleic acids).
Differential diagnosis of turbid peritoneal effluent.
| Time to appearance | Pain intensity | Appearance of effluent | Cellularity | Keys to diagnosis | |
|---|---|---|---|---|---|
| Peritoneal infection | Variable (includes inactive PD) | +/++++ | Turbid | 100/>25,000 leukocytes/mm3>50% PMN | CultureCellularityDefault diagnosis |
| Eosinophilic peritonitis | Early | 0/+ | Mild turbidity(often intermittent) | <500 leukocytes/mm3>10% eosinophils <50% PMN | TimingCellularity |
| Peritonitis associated to icodextrin | Variable | 0/++ | Mild to moderate turbiditySometimes intermittent | 100−1,000 leukocytes/mm3Monocytosis | Icodextrin Presentation as outbreaksCellularity |
| Chyloperitoneum | Variable | 0 | MilkyTurbid if mild | <<100 leukocytes/mm3 | AppearanceTriglycerides in effluentStudy of supernatant |
| Hemoperitoneum | Variable Recurrent | 0/++ | HematicTurbid if mild | Erythrocytes<100 leukocytes/mm3 | Cellularity |
| Fibrin-rich effluent | Variable | 0 | TurbidThrombus formation upon resting | <<100 leukocytes/mm3 | Resting of bagCellularity |
| Neoplasm | VariableEarly | 0 | Mild turbidity | VariableAtypias | Cellularity (cytology) |
| Chemical peritonitis | Variable | 0/++++ | Turbid | 100/>10.000 leukocytes/mm3Variable PMN | Causal agent |
| Empty peritoneal cavity | Variable | 0 | Mild turbidity | 100−200 leukocytes/mm3 Monocytes | Starting PDNocturnal intermittent PDCellularity |
| Pigments | Variable | 0 | Light colored | <<100 leukocytes/mm3 | JaundiceMedication |
In a small percentage of patients, PI may initially manifest as abdominal pain but with a clear effluent. In these cases, it is advisable to repeat the exchanges with a minimum dwell of two hours, monitoring the appearance of the dialysate. A diagnostic study is always indicated in the case of doubt or suspicion.
Nomenclature of peritoneal infectionA series of definitions have been established for the standardized classification of certain PIs according to the context in which they appear and their clinical course (see Table D6).
Basic nomenclature of peritoneal infections.
| Repeated infection | Episode appearing more than 4 weeks after completing treatment of a previous episode caused by the same organism. |
| Relapse | Episode appearing less than 4 weeks after completing treatment of a previous episode caused by the same organism (or negative culture) |
| Recurrent infection or new PI | Episode appearing less than 4 weeks after completing treatment of a previous episode caused by a different organism |
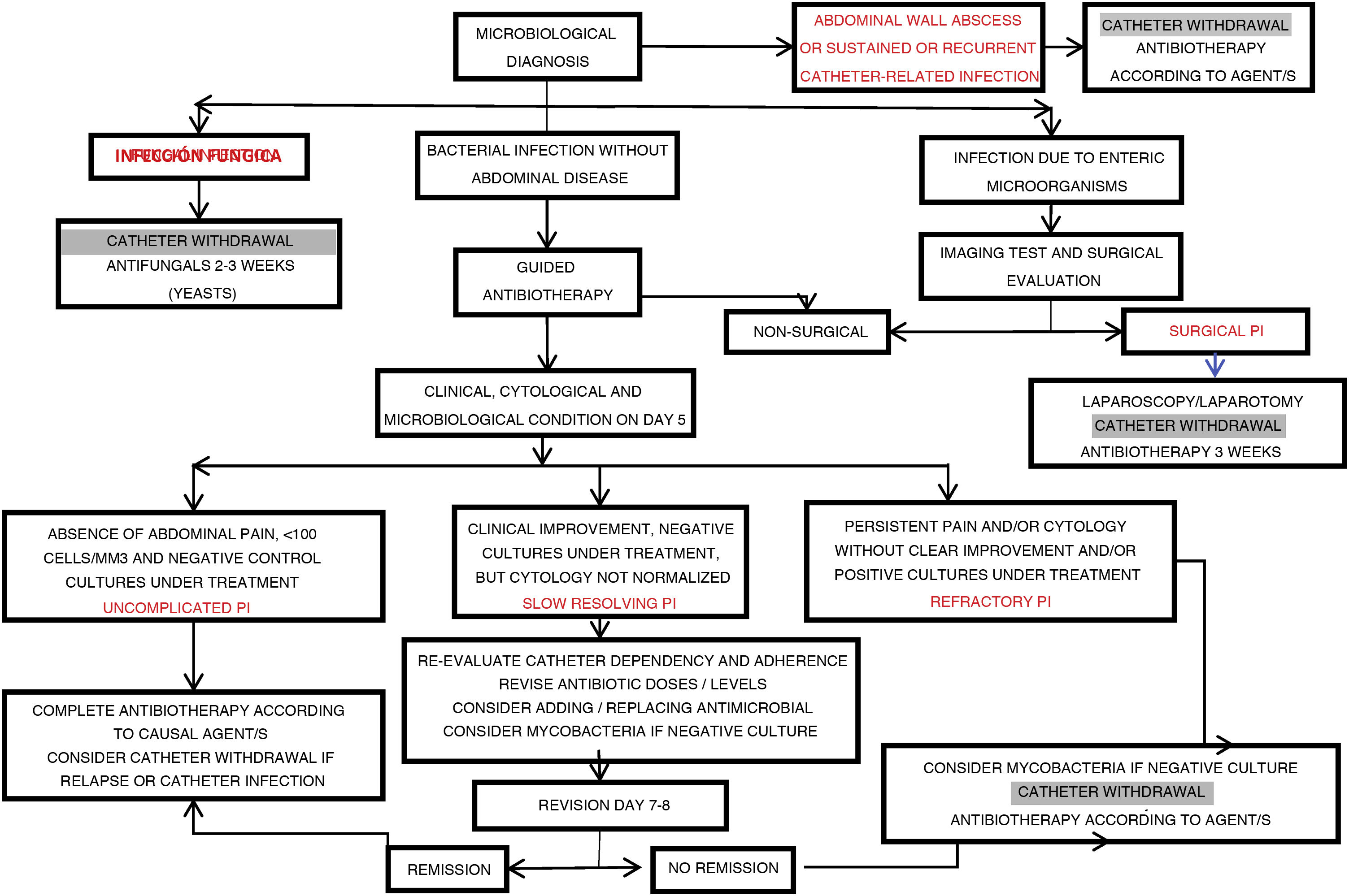
| Slow resolving infection | Episode showing a clear tendency towards clinical and cytological improvement (with negative control cultures), but maintaining signs of activity after 5 days of adequate antibiotic treatment |
| Refractory infection | Episode showing no evident signs of resolution after 5 days of adequate antibiotic treatment |
| Enteric infection | Episode showing an underlying gastrointestinal or hepatobiliary infectious site, orEpisode with the isolation of at least two enteric microorganisms (enterobacteria, enterococci and/or intestinal anaerobes), orEpisode with isolation of an intestinal anaerobe(It is subject to debate whether the isolation of a single enteric organism is able to establish the same diagnosis) |
| Catheter-related infection | Episode coinciding with exit site infection or infection of the tunnel caused by the same microorganism |
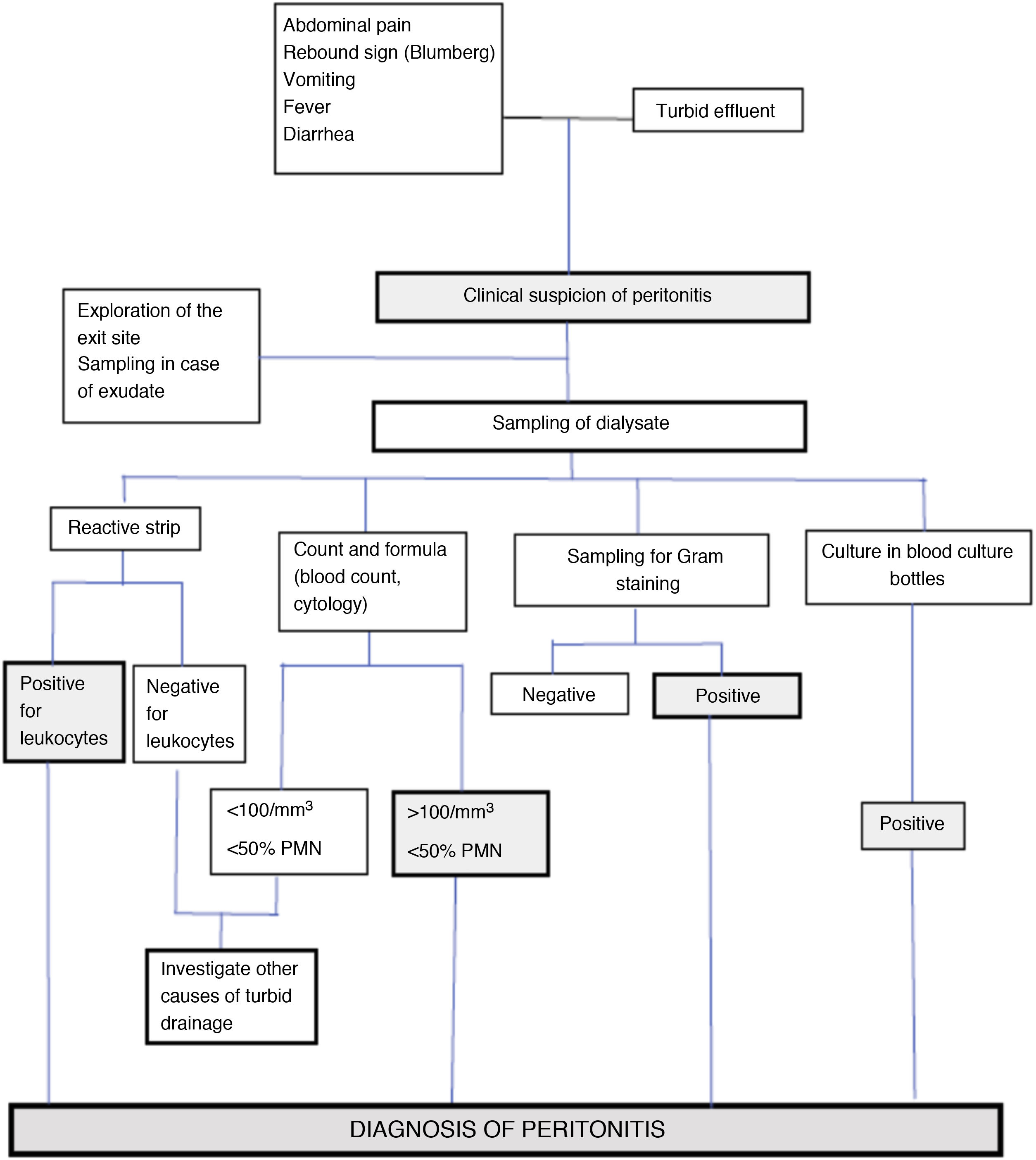
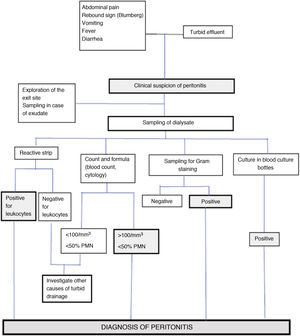
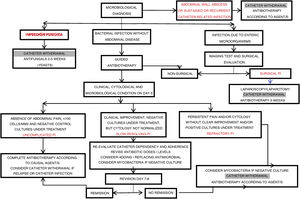
The diagnostic process of PI (Fig. D2) usually starts when the patient consults because of some of its cardinal manifestations (abdominal pain and/or the appearance of a turbid effluent).
The initial aims in the event of a probable PI are to:
- 1)
Establish the diagnosis
- 2)
Compile information on the underlying etiopathogenesis (contamination route and probable causal microorganism)
- 3)
Detect aggravating factors
- 4)
Assess the clinical condition of the patient
Application of the clinical method is the best way to start assessment:
- -
Anamnesis (case history) to identify remote or recent data of interest: age, comorbidity, previous abdominal processes, previous infections (peritoneal or catheter-related), recent antibiotherapy.
- -
Data immediately related to the event, compiled through a non-inculpatory interview: warning manifestations, breaching of the technique, possible bacteremic procedures, etc.
- -
General physical examination, with special attention to signs of impaired general condition (sepsis), peritoneal irritation and catheter-related infection.
- -
Inspection of the bag. Patients with suspected PI usually report to the center with the abdomen full of fresh dialysate (introduced in the abdominal space after detecting the turbid drainage) or following variable dialysate dwells, when the abdominal manifestations cause them to consult without performing the exchange. In the former scenario it is advisable to analyze both the bag brought from home with turbid dialysate and the dialysate drained in the center. This double sampling increases the chances for rapid diagnosis and allows us to assess the initial evolution of the situation.
Adequate retrieval and processing of the samples is essential in order to ensure a high rate of valid results14,55.
Retrieval/collection- -
Sufficient dialysate volume (>50 ml) is extracted using an aseptic technique.
- -
The sample is transported in a sterile container without preservative.
- -
Part of the collected sample is inoculated in blood culture bottles (5−10 ml/aerobe and anaerobe bottle). A tube is to be reserved for direct Gram staining of the sample.
- -
Until transport to the laboratory, the samples are to be stored at under 4 °C (except the blood culture bottles, which are to be kept at room temperature).
- -
Whenever possible, the samples are to be processed within 6 h. A delay of over 12 h can reduce the reliability of the results.
- -
From the practical perspective, a number of studies have shown that direct inoculation of the dialysate sample in blood culture bottles simplifies the procedure and affords successful results in a short period of time.
- -
In centers with high negative culture rates, the results can be improved by centrifuging the effluent and culturing the resulting sediment in both solid media and inoculated in blood culture bottles.
If the cultures prove negative after 3−5 days using blood culture bottles, but the clinical evidence is strongly suggestive of PI, subcultures should be made on agar plates incubated at 37 °C in an atmosphere with 5−10% CO2 and in an anaerobic atmosphere, during an additional 3–4 days, in order to identify difficult-to-grow microorganisms.
AnalysisThe samples of peritoneal effluent are processed in two ways (Fig. D2):
- Study of cellularity – Leukocyte count: Analysis of the leukocyte count in dialysate requires a sample dwell time of at least two hours14. There is no evidence that samples obtained after very long peritoneal dwell times may give rise to overdiagnosis, though it is advisable to individualize the interpretation of cellularity in samples obtained from empty peritoneal cavities or in cases with very small drainage volumes. A low leukocyte count in the presence of marked turbidity suggests the associated presence of fibrin, other proteins, lymph or hemoperitoneum (Table D5).
The leukocyte differential formula is useful in situations where the dialysate sampling conditions are suboptimal due to short dwells in the abdominal cavity, or the use of a diluted sample. In these situations, the presence of >50% of PMNs supports the diagnosis. A relative eosinophil count of >10% establishes the diagnosis of eosinophilic peritonitis. Mild to moderate eosinophilia in PI can be seen in sporadic cases56, particularly in fungal or parasitic infections, as well as in association to chemical irritants (e.g., vancomycin), or the use of icodextrin. However, the presence of marked eosinophilia does not suggest an infectious origin of the condition.
The reactive strips commonly used for urine testing may be useful for the early diagnosis of PI57. These strips detect the presence of leukocytes, though in qualitative terms and without providing a differential count, with a sensitivity of close to 100%, a specificity of 95–97%, and a positive predictive value of approximately 95%. Their advantages include the immediate obtainment of results, and low cost. The study of the peritoneal effluent sediment based on the modified automated blood count is widely employed, since it is reliable, rapid and inexpensive. Conventional cytology (Giemsa and Papanicolaou staining) is even more reliable, and allows us to assess atypical presentations (e.g., neoplasms), but such techniques are expensive and should only be used where specifically indicated.
- Bacteriological study. The study of a dialysate sample subjected to Gram staining is very recommendable, despite its low sensitivity (which can be incremented if the sediment is resuspended after centrifugation of the original sample), since it is strongly orientative when positive14. In particular, the presence of yeast forms is usually easy to detect with this method58.
Microbiological culture identifies the causal microorganism and guides the best therapeutic option.
Complementary diagnostic methods in peritoneal infection- -
Imaging tests: Imaging techniques contribute little to the routine evaluation of PI in PD, and in most cases are not needed to confirm the diagnosis. However, they may be required when an underlying intraabdominal process is suspected. The most frequent scenarios are:
- -
After identification of mixed flora, either at the start of the diagnostic process (Gram staining), or through isolation in culture over the subsequent days.
- -
Aggressive clinical presentation, particularly in the presence of signs of sepsis. The intensity of pain is generally less reliable, since it is a subjective manifestation. Some unconsequential infections may initially manifest with intense pain59.
- -
Presence of localizing signs at physical examination.
- -
Clinical antecedents suggesting a highly likely secondary origin of the infection (e.g., recent acute cholecystitis).
- Laboratory tests: Table D7 shows the main complementary tests proposed for improving the diagnostic efficacy of PI60–67. Of all of them, the detection of mycobacteria through PCR testing of peritoneal effluent is the only option that has become widely consolidated in clinical practice.
Other procedures for the diagnosis of peritoneal infection.
| Diagnostic test | Usefulness | Reference |
|---|---|---|
| Detection of microbial DNA fragments | Predictor of relapse | 58 |
| Sequencing of ribosomal RNA | Allows the detection of occult germs additional to the main isolate | 59 |
| Detection by polymerase chain reaction (PCR) techniques | Complement to microbiological cultures. Mycobacteria | 60 |
| Nitric oxide dialysate/plasma (D/P) ratio | Severity of infection and response to treatment | 61 |
| Determination of matrix metalloproteinase 9 (MMP-9) levels | Equivalent to peritoneal leukocytosis | 62 |
| Immune fingerprints | Early identification of the causal pathogen | 63 |
| Determination of adipokines in peritoneal effluent | Diagnosis of peritonitis | 64 |
| Determination of endotoxins | Infection with an atypical course | 65 |
The general criteria applicable to PD are to be followed. However, patients on APD have particular characteristics that can complicate an early diagnosis:
- The frequent nocturnal exchanges, with short dwell times, generate a peritoneal lavage effect that can attenuate clinical expression, reduce the cell count in the dialysate and dilute the concentration of microorganisms. In contrast, the long dwell times of diurnal exchange can result in relatively high cell counts that can give rise to doubt — though in this case the predominance of monocytes helps to exclude the presence of PI.
- There may be a delay in diagnosis, since in some cases turbidity of the drained fluid is not observed until the next morning.
- The type of container used to hold the drained dialysate may also interfere with the diagnosis. Cyclers usually drain the effluent directly to the wastewater system or to collector bins in which the appearance of the drained fluid is difficult to evaluate (furthermore, the traces of detergents used to clean them can cause turbidity without an infectious origin). In the case of suspicion, transparent collector bags (designed for this purpose) are the best option for examining the dialysate.
The general recommendation to obtain the dialysate sample after a minimum dwell time of two hours (whenever possible) is maintained. However, patients on APD raise a number of possible scenarios:
- -
The patient presents suspicious clinical manifestations at the start of the nocturnal session. In this case it is advisable to obtain a sample of the effluent of the long exchange in the corresponding sample collection bag, which the patient will take to the center for processing. If the evidence of infection is clear, the patient may opt to directly report to the center.
- -
If the clinical manifestations appear during the cycling procedure, the usual practice it to complete the session and report to the center once it has been completed, to confirm the diagnostic suspicion, collect samples and start management. If the initial manifestations prove aggressive, it is advisable to suspend the session and report directly to the reference center.
- -
If manifestations appear during the diurnal period, the patient should perform manual exchange and, if turbidity is confirmed, he or she should report to the center with the drained bag. If the patient has not been trained to perform manual exchanges, he or she should report to the center directly.
- -
Once PI has been diagnosed and treatment has been started, it is essential to monitor the course of the infection. If the patient is not admitted to the center, a clinical check (preferentially on site) should be made 48−72 h after the start of the episode, followed by subsequent checks with the same frequency until clinical improvement is confirmed. These checks should include cellularity studies. While the entire panel of experts considers it necessary to perform control cultures in the presence of a poor clinical course, the decision regarding the need for such measures in PI evolving without incidents is subject to debate (50% do not consider them necessary while 41% do consider them necessary). No recommendation therefore can be made on this issue in the present guide. The follow-up and assessment of the diagnostic tests allows the detection and specific management of infections of an atypical nature or course (Table D6).
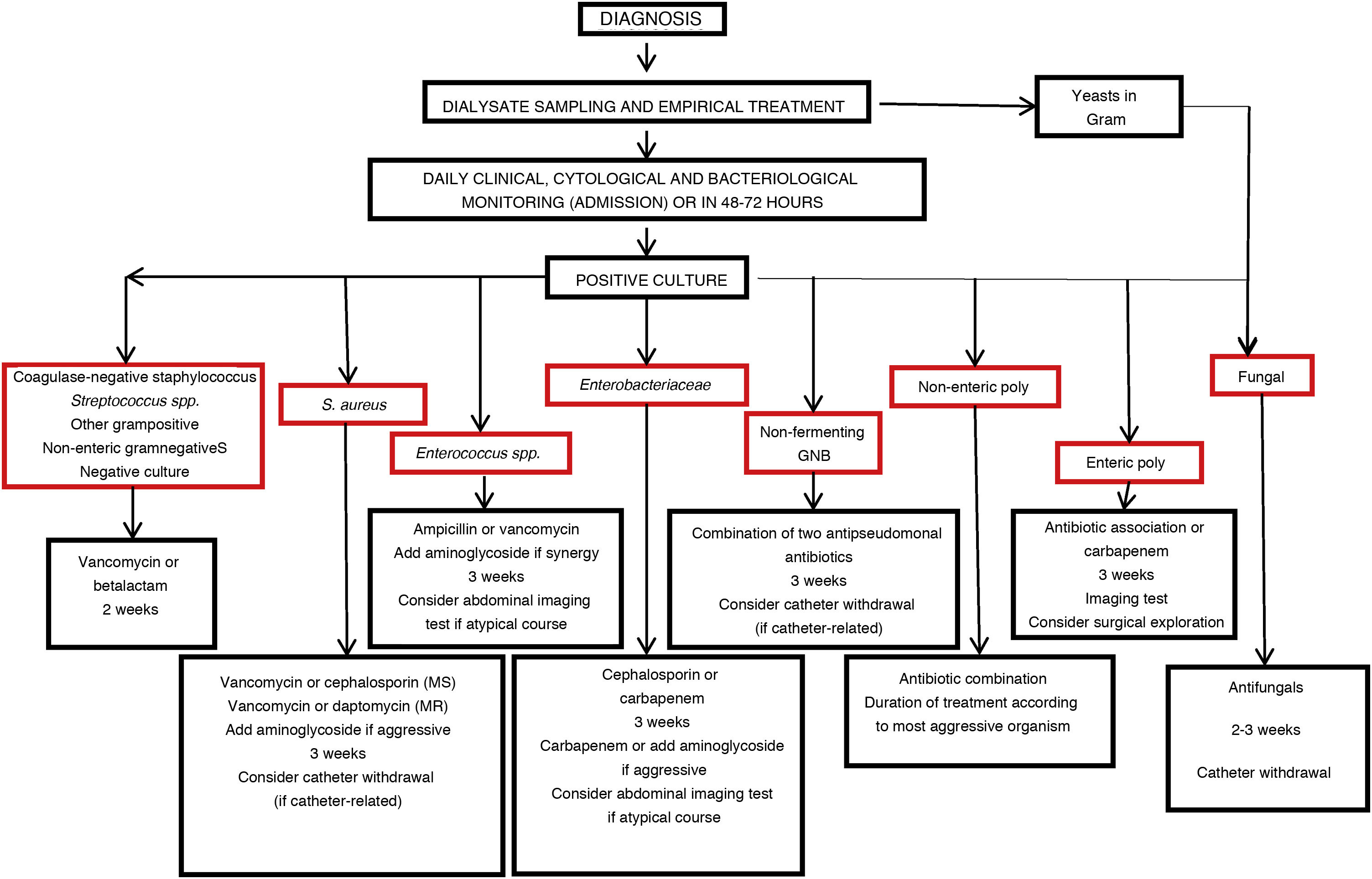
If no favorable response is observed on the fifth day of specific treatment, the dialysate culture should be repeated, expanding the search to include uncommon pathogens. The incidence of unresolved or complicated cases of PI is very high in those cases where the cell counts remain elevated after 5 days of treatment (slow-resolving or refractory PI).
It is advisable to periodically check the results of the baseline and follow-up cultures, since there may be late growth (anaerobes, yeasts, filamentous fungi), and masking may occur (the eradication of one microorganism may reveal an underlying second pathogen).
Conclusions regarding the diagnosis and terminology of peritoneal infection (ungraded)- 1)
Peritoneal infection should be suspected in the presence of abdominal pain and/or a turbid (cloudy) peritoneal effluent. The diagnosis is to be confirmed by cytological and microbiological study of the effluent.
- 2)
An abnormally high concentration of leukocytes (>100/mm3) with a dominant presence of PMNs (>50%) in the effluent is strongly suggestive of PI.
- 3)
The diagnosis of PI is to be individualized in relation to the clinical circumstances, fundamented upon the criteria described in the above sections.
- 4)
Careful initial assessment, with correct collection and processing of the samples, is essential for the successful management of PI.
- 5)
A systematic microbiological analysis of the effluent based on Gram staining, within the initial diagnostic process of PI, is strongly recommended.
- 6)
Each center should compile evolutive and up to date information on the PI rates (overall and according to causal pathogens), using standardized estimators, and at least once a year.
- 7)
Ideally, the monitored parameters should include the overall PI rate, the PI rates according to specific microorganisms, the percentage of patients/year that remain free of PI, and the antimicrobial resistances of the causal pathogens.
- 8)
The PI rates are best reported as the number of episodes per patient and year.
- 9)
Similar PI diagnostic criteria should be applied in patients on either manual or automated PD.
- 10)
It is crucial to systematically monitor PI through clinical, cytological and microbiological controls, until a clearly favorable course is confirmed.
The prevention of PI is a fundamental objective in PD, due to its great clinical impact and effect upon survival of the patient and of the PD technique, quality of life, economical costs and the perception of the technique itself. Many measures have been proposed over the last 40 years for the prevention of such infections. In order to analyze these measures from an updated perspective, it may be useful to classify them according to their impact upon the main recognized routes of contamination that culminate in PI:
- -
Touch contamination (intraluminal route)
- -
Catheter-related (intra- and periluminal routes)
- -
Hematogenous spread (bacteremic route)
- -
Contiguity spread (enteral, biliary and gynecological routes)
Accordingly, we may establish the following types of preventive measures that will be analyzed in the following sections:
- 1
Multiple or general impact measures
- a
Adherence to the recommendations of the clinical practice guides
- b
Structured preventive strategies
- c
Patient training
- d
Management of carriers
- a
- 2
Measures aimed at preventing catheter-related infection (CRI)
- a
Catheter design
- b
Insertion technique and immediate management
- c
Care of the exit site
- a
- 3
Measures for the prevention of touch contamination
- a
Connectology
- b
Automated techniques
- a
- 4
Improvement of peritoneal antiinfectious defense mechanisms
- 5
Special situations and secondary prevention
The advisability of strict adherence to the recommendations of the present guide or to those of other clinical practice guides referred to PI is debatable, given the scarcity of firm evidence on the questions raised. Most recommendations are based on the opinion (even when of a collegiate and fundamented nature) of groups of experts, who can be more or less accurate in the same way as any other groups of experts. It may prove difficult to convince a team with extensive experience in PD to follow the recommendations of professionals who are no more experienced than themselves, and which may relate to questions under clinical and sociosanitary circumstances that differ from those of their own particular setting. Furthermore, there is no evidence that strict adherence of each and every one of the recommendations of the guides is able to improve the outcomes, though there are clear signs — particularly from the Anzdata setting44,68 — that structured interventions based on the principles contained in the guides are able to improve the control of PI, and initiatives have been made seeking to confirm these signs69.
Overall, this Committee expresses the following OPINIONS (ungraded):
- 1
Any clinical guide on the prevention of PI with quality criteria (directed by groups of recognized experts and using standardized methods) can provide useful guidance for the prevention and management of PI.
- 2
The strength of the recommendations is conditioned by the quality of the evidence, but also by the consistency of the results of previous studies on the topic. In general, recommendations based on quality evidence and/or strong recommendations should be followed.
- 3
Suggestions, based more on opinion than on evidence, should be considered by those centers lacking clear experience in the subject at hand, while experienced groups must have more freedom to adapt to their particular circumstances.
- 4
The maximum benefits are expected more from global adherence to the clinical practice guides than from the adherence to concrete recommendations of such guides.
In recent years there has been growing interest in the application of strategies for continuous quality improvement (CQI) in healthcare practice, and PD has been no exception in this sense43,70,71. These strategies imply a global and continuous approach to problems (in our case, the general outcomes of a PD program), identifying and correcting the weak points in order to be able to detect and mitigate the weaknesses of the system. This process must follow a series of steps that include the correct definition of objectives, the designing of clear and concise protocols, adaptation to the available resources and, fundamentally, the monitoring of results71. In the concrete case of PI, any initiative for improvement is critically dependent upon detailed and up-to-date knowledge of the incidence of this complication. The ISPD recommends monitoring the events on a standardized and iterative or continuous basis11. This approach allows periodic comparison of the results, and the identification of effective preventive practices to ensure continuous improvement of the outcomes.
The ISPD guide of 2016 addresses the impact of continuous quality improvement strategies upon the incidence of PI in PD to only a limited extent14. The apparent reason for this is the lack of publications on the subject, and the existing studies are moreover of an observational and uncontrolled nature, and with evident risk of publication bias. Nevertheless, the studies coincide in detecting very significant reductions in the incidence of PI72,73. Other studies, involving prospective and more orthodox designs, have ratified these results70,74.
Little additional evidence has been forthcoming since the publication of the ISPD guide of 201614. An Anzdata document68 has analyzed the potential measures for consistently reducing the incidence of PI in PD, from a continuous quality improvement approach. In particular, the document underscores the convenience of the following:
- 1
Facilitation of the awareness and disclosure of poor outcomes in comparison with other Units
- 2
Identification of the needless variations in updated good practices
- 3
Adaptation of the clinical practice guides to improve those areas with poor outcomes
In addition, it is recommended that scientific societies, patient associations and the public institutions should collaborate to:
- 1
Define and establish operating and outcome indicators in the PD Units
- 2
Conduct periodic audits
- 3
Compare outcomes and practices between Units in order to adopt and implement improvements
- 4
Favor the existence of registries, which should include data reflecting the level of care quality in the PD Units
- 5
Establish a minimum body of professional standards
- 6
Favor training programs, especially for residents in training and nurses dedicated to PD
- 7
Identify the evidence shortcomings in clinical practice and adopt measures to correct them
Makhija et al.75 recently published an analysis of the economical benefits of a continuous quality improvement program, fundamentally resulting from a reduction in the incidence of PI. Their estimations, based on real data, included an investment return of 169% (the cost of carrying out the program minus the savings derived from the reduction of the number of events). The main measures of the continuous quality improvement process were improvement of the nurse/patient ratio, the adoption of exit site care protocols, standardized patient training guides, ongoing training and certification of nurses dedicated to PD, home visits and support, and the monitoring of results, with the adoption of corrective actions when needed. This study evidences the need for the organization and health authorities to be motivated to economically invest in actions for improvement43.
Another study, also from Colombia76, analyzed the incidence of early PI (in the first 90 days) in a national structure (3525 patients from 49 Units), where care was organized around highly standardized and evidence-based practices. The study reported very acceptable infection rates (0.23 episodes per patient and year), which the authors attributed to the integral and standardized prevention strategies used. The retrospective design of the study, the absence of well-defined prior objectives, and the lack of a control group weaken the conclusions drawn, however.
Quality of evidence: Low
Synthesis of the evidence
This question has been addressed in a succinct manner by the ISPD guide of 201614. In essence, the recommendations are based on scarce and low-quality evidence, as well as on analysis of strategy documents. No additional quality evidence has been forthcoming since the ISPD guide of 2016, though there has been a cost-efficiency analysis of some healthcare interest75.
From evidence to recommendation
This Committee supports the recommendations of the ISPD guide of 201614.
Recommendations
- -
We recommend each PD Unit to develop a Continuous Quality Improvement program in order to reduce the incidence of PI (1C).
- -
We suggest periodic meetings of the multidisciplinary teams in charge of the continuous quality improvement program in order to analyze the outcomes (2C).
The ISPD guide of 2016 makes reference to other guides of the same organization11,77, and particularly to an update from the same year 201624, to define its recommendations regarding the role of PD patient training and monitoring. Although these recommendations are of a general nature, the prevention of infections remains their main focus. The most important recommendations are referred to:
- -
The convenience for training and monitoring to be carried out by nursing staff trained in both PD and in the education of patients.
- -
The need for standardized training protocols well focused to targets (in this case, the prevention of infections). In practice, these protocols are very much centered on the prevention of PI associated to CRI and touch contamination.
- -
The importance of checking the skills acquired by the patient during the training process.
- -
The advisability for the staff in charge of training to carry out at least one visit to the home of the patient. This type of activity allows us to detect situations of risk, inconsistencies and breachings of protocol that are not noticeable in the center, and is considered to be useful even though the evidence of its benefits in terms of lowering the incidence of PI is weak78. The capacity of televisiting (remote visiting) to replace face-to-face visits when the latter are not possible, has not been established.
- -
The usefulness of patient retraining in order to correct errors, deviations from protocol and adherence problems. Depending in part on the available resources, some centers only intervene in concrete circumstances (prolonged hospital admission, CRI or PI, changes in the physical condition of the patient, changes in supplier or interruption of PD for prolonged periods)14, while other centers resort to programmed periodic retraining. Here again, the evidence on the benefits of this measure in relation to the risk of PI was weak at the time of publication of the guide79,80.
One of the most interesting aspects is the impact of the experience of the trainers and the time dedicated to patient training. A retrospective study of the BRAZPD multicenter group81 analyzed the training of 2243 patients, and found the dedication of over one hour a day and over 15 h in total to be associated to a lower risk of PI. Similar benefits were observed in centers with greater experience among treated patients and when training was started even before insertion of the catheter. In contrast, the number of trained individuals (including care givers) did not influence the observed risk. In this same line, the experience of the PDOPPS group5 suggests significant benefit when training is prolonged for over 6 days. A survey of the French registry on 5017 patients in 127 Units82 showed centers with nursing staff dedicated to PD and the conduction of home visits before the start of dialysis to be associated to lower PI rates, while the activity of the Unit had no apparent effect. Remarkably, another later survey by this same registry83, involving 1035 patients in 94 Units, did find those centers with greater experience (over 10 prevalent patients) to have a lower incidence of PI. Furthermore, basing training only on written texts, or contrarily only on the practice of dialysis exchange, was associated to a greater risk of infection.
A controlled trial84 observed no global benefit of more frequent retraining in terms of the risk of PI, though a marked decrease in risk among elderly patients was noted.
A more recent trial conducted in China85 randomized 150 patients to retraining under direct monitoring by the staff, retraining through a verbal questionnaire, or no retraining. The group under direct monitoring had lower PI rates (0.13 episodes per patient and year) than the other two groups (0.19 and 0.17 episodes per patient and year, respectively) (p < 0.005).
A third multicenter and better powered trial86 in turn randomized 671 patients to periodic retraining (on each scheduled visit, in the case of PI, or after suspending PD for more than 6 weeks) including practical tests, or to control without retraining. The risk of PI was found to be no different between the two groups.
Initiatives have been made to determine which training practices are most effective, in a quest to establish unified protocols87–89.
Quality of evidence: Moderate
Synthesis of the evidence
This question has been addressed in a succinct manner by the ISPD guide of 201614, with reference to other guides of the same organization11,24,77. With few exceptions, the recommendations are based on opinion. Several important studies have subsequently been published. The observational studies, mostly based on registries, mainly (but not exclusively) suggest that the acquired experience (established from the number of patients treated) and time dedicated to training have a positive effect upon the ulterior risk of PI5,81,83; that specialized nursing staff achieves better outcomes82; and that home visiting before the start of treatment offers similar benefits82. On the other hand, the results of randomized trials have been disappointing regarding the efficacy of retraining in reducing the PI rates84,86 – though a recent trial83 has evidenced benefit when retraining is made under direct monitoring.
From evidence to recommendation
The recent evidence does not warrant changes in the current guidelines of the ISPD14,24, which this Committee supports.
Recommendations
- -
We recommend the patient training and monitoring principles proposed by the documents of the ISPD in order to reduce the PI rates (1C)
- -
We recommend the nursing staff in charge of patient training and monitoring to be specialized (1C)
- -
We suggest that visits to the home of the patient, if possible beginning before the start of treatment with PD, may help reduce the risk of PI (2C)
- -
We suggest that retraining under indication could help reduce the incidence of PI in situations of risk (2C)
- -
Based on the available evidence, this Committee does not endorse programmed retraining for reducing the incidence of PI (2B)
The prevalence of Staphylococcus aureus nasal carrier status in the general population is variable, though it has been estimated that about 20% are persistent carriers, 30% are intermittent carriers, and 50% are resistant to colonization by this microorganism89. The percentage is probably similar among patients on PD90–92. It is known from the first studies on the subject93,94 that the efficacy of intranasal mupirocin for the prevention of PI due to Staphylococcus aureus is less consistent than that observed when the drug is administered at the catheter exit site (see below). Two recent meta-analyses95,96 have shown that this specific measure does not reduce the risk of PI. Nevertheless, assuming that a reduction in the incidence of CRI is associated to a decrease in the risk of PI, and considering the benefits of this strategy in patients on hemodialysis 97 and the small number and limited statistical power of the available studies in PD, it is not possible to rule out a beneficial effect of this measure. The ISPD guide of 2017 25 endorses the administration of intranasal mupirocin in nasal carriers of this microorganism, though with the purpose of preventing CRI.
Prevention of peritoneal infection secondary to catheter-related infection (CRI)Catheter-related infections are a relevant complication of PD25, and are associated with a significant risk of PI98,99. This association may be of a pathological nature (when CRI progresses towards the peritoneal cavity through the catheter lumen or via the peri-catheter route), or can reflect a general patient predisposition towards infection (e.g., frail individuals, with poor adherence to the technique, or carriers of Staphylococcus aureus). The prevention of CRI has been the subject of a recent document of the ISPD25, and a thorough review of this topic goes beyond the scope of the present guide. Based on the premise that any measure capable of reducing the risk of CRI will afford protection against PI to one degree or other, the present chapter focuses on the potential role of certain specific measures for the prevention of PI related to catheter insertion and care.
Measures related to design and insertion of the peritoneal catheterDespite the lack of direct evidence, it seems reasonable to assume that correct insertion and early care of the peritoneal catheter will lessen the ulterior risk of CRI and, therefore, of PI. It is advisable to carefully select the cutaneous insertion zone and exit site of the catheter (avoiding folds or friction zones), prepare the surgical field in accordance with the general surgical principles, and observe strict asepsis during insertion. Alternative strategies have been developed for patients in which insertion in conventional zones poses a problem (extreme obesity, stomas). These strategies make PD feasible, but their benefits in terms of the prevention of infections have been generally disappointing100.
The technique for construction of the catheter exit site is another potentially relevant issue. The cutaneous orifice should be relatively well adjusted to the catheter itself, and should be located at least 1 cm (and preferably 2−4 cm) from the surface external Dacron cuff (in catheters with two cuffs). Some experiences101 have led to the suggestion that orienting the catheter exit site downwards and avoiding suture stitches close to the orifice reduces the risk of CRI and of secondary PI11,25, though the evidence in this respect is inconclusive102,103.
Catheter designThe ISPD guides of 2016 offer no specific recommendations regarding the impact of the type of catheter upon the risk of PI14. Neither the internal (straight versus pigtail) nor the intraparietal morphology (straight versus swan neck), or the number of Dacron cuffs (one or two), appear to influence the incidence of this complication. In relation to this issue, it should be mentioned that a significant number of randomized trials have been published (and referenced in the guide14).
Following the publication of the guide, a number of further publications of interest have appeared. In a clinical trial, Sánchez-Canel et al. randomized 78 patients to two types of catheters with a Dacron cuff: one with a simple straight design and the other with a self-positioning design104, with no observed differences in the risk of PI. The randomized trials of Ouyang et al.105, Banin et al.106 and Chow et al.107, involving catheters with a straight or pigtail intraperitoneal portion, evidenced no differences in the risk of PI - though the first infection was detected earlier in the patients with a straight catheter (mean 6.5 months) versus a pigtail catheter (11.7 months) (p = 0.007), in the study published by Ouyang105. The recent studies thus support the previous information, though it must be noted that, in all cases, the risk of PI was a secondary outcome variable, and that most publications did not even comment on the results referred to this matter.
Peritoneal catheter insertion techniqueThe ISPD guides of 2016 makes only limited mention of the influence of the catheter insertion technique upon the ulterior risk of PI. Although the evidence available up to the time was of reasonable quality, the rather inconclusive results did not allow any recommendations to be made regarding the possible advantages of laparoscopic insertion versus open insertion, the medial approach versus the paramedial approach, presternal versus abdominal insertion, or burying of the catheter using the Moncrief method108 versus conventional management14,109.
Following the appearance of the 2016 guide, a number of systematic reviews and meta-analyses have been published110–114 seeking mainly to clarify the possible advantages of laparoscopic insertion of the catheter versus insertion through puncture or the open technique (minilaparotomy). In general, no differences have been found in terms of the risk of PI. The most extensive and complete analysis is possibly that published by Htay et al.113. This study evidenced no differences in the risk of PI in relation to the insertion technique used (laparoscopic versus open), burial of the catheter, or the insertion zone (medial versus paramedial).
Antibiotic prophylaxis before or simultaneous to insertion of the peritoneal catheterThe ISPD guides of 2016 underscores the benefits of antibiotic prophylaxis in the insertion of the peritoneal catheter, in terms of the risk of PI14. This observation was based on a significant body of studies which, apart from some exceptions115, evidenced that different antibiotic prophylaxis regimens are superior to no treatment116–118. The use of antibiotic prophylaxis was subsequently endorsed by a systematic review published in 2004, which showed its benefits in terms of lowering the risk of PI, but not of CRI119,120. With regard to comparison of the different regimens, a randomized trial demonstrated superiority of vancomycin over cefazolin118, though the latter drug is still often used.
Since the publication of the ISPD guides of 2016, little further evidence has been forthcoming on this subject. In a clinical trial, Velioglu et al.121 compared cefuroxime via the oral route (500 mg every 12 h, starting pre-insertion and continuing for 3 days) and the administration of cefuroxime via the intravenous route (a single pre-insertion dose of 750 mg). The cumulative infection rate after two weeks was 9.0% and 8.1%, respectively (p = 0.578). The study included an analysis of the costs, which proved somewhat higher for the intravenous route, but were acceptable in both arms of the study. A recent updated review on the prevention of infections in PD122 has afforded no additional evidence in relation to pre- or perioperative prophylaxis. The results of the prospective PDOPPS study5 have confirmed that those centers which administer antibiotic prophylaxis for insertion of the catheter have a lesser incidence of PI (relative risk [RR] 0.83, 95% confidence interval [95%CI] 0.69−0.99).
Quality of evidence: Moderate
Synthesis of the evidence
This issue has been adequately addressed by the ISPD guides of 201614, based on randomized studies which, despite the negative results, offer a sufficient level of evidence, supported by a number of subsequent systematic reviews and meta-analyses110–114. Recent randomized trials have provided confirmatory evidence, particularly as regards the comparison of catheters with different designs of the intraperitoneal portion104–107.
From evidence to recommendation
This Committee supports the recommendations of the ISPD guides of 201614.
Recommendations
- -
We recommend the administration of prophylaxis with systemic antibiotics immediately before insertion of the peritoneal catheter (1A)
- -
We suggest that each PD program should choose its own antibiotic prophylaxis regimen, considering the local incidences and antibiotic sensitivities (2D)
- -
We suggest vancomycin as a preferential option, though first or second generation cephalosporins may afford equivalent results (2B)
- -
We recommend that the risk of PI should not be a consideration when deciding which insertion technique or peritoneal catheter design to be used in a patient on PD (1A)
The risk of CRI, and secondarily of PI, may also depend on the quality of care after insertion of the catheter, over both the short and long term.
Care of the exit siteThe ISPD guide does not focus much on the subject of the care of the catheter exit site, apart from establishing a generic recommendation to observe careful hygiene. This issue was subsequently addressed much more in depth by the ISPD guide on the prevention of CRI25. The only recommendation of the mentioned guide is that care of the exit site should be made twice a week and whenever the patient showers. This recommendation is not supported by controlled evidence. In the opinion of the great majority of the panel of experts of this guide (81%), daily care should be the standard practice, in our setting. Other considered options, but with no specific recommendations due to the lack of evidence, include25 adequate immobilization of the catheter in order to reduce traction-induced trauma, short cycles of local or systemic antibiotherapy in the case of damaged exit sites, and the use of isolation systems (specific or simple colostomy bags) for patients that practice swimming123. The type of care best suited for the exit site of the catheter likewise has not been well defined. Although generic or PD-specific protective systems are used, there is no evidence of the superiority of one specific option over the rest. The impact which any of these measures may have upon the risk of PI is not known. An interesting recent randomized trial124 has compared the convenience of care with or without a dressing applied to the exit site, in a setting characterized by a low incidence of infection. The PI rate was found to be similar in both groups, though a notorious finding was that the open orifice care group presented longer time periods to the first PI episode (250 days) than the closed orifice care group (98 days) (p = 0.03). It must be noted that the mentioned study was carried out in a warm and humid environment — a scenario that is not fully extendable to the situation found in Spain. A retrospective study, with a low level of evidence125, recorded PI rates up to 9-fold higher after closed peritoneal catheter orifice care than following open care.
The effect of tunneling the catheter following its insertion has not been clearly established. We have already mentioned that burying the catheter using the Moncrief technique108 does not appear to reduce the ulterior risk of PI. A recent randomized trial126 has compared the incidence of mechanical and infectious complications according to whether PD is started one, two or four weeks after insertion of the peritoneal catheter. The study, with a limited statistical power, detected no differences in PI rate.
Use of disinfectants and antibiotic prophylaxisBoth the ISPD guides of 2016 on PI14 and the guide of 2017 on CRI25 examine this issue in detail. The salient aspects, which coincide considerably in both guides, are:
- -
There is no evidence of the superiority of any of the different disinfectants used for the routine care of the peritoneal catheter exit site (hydrogen peroxide, hypertonic saline solution, soaps or antiseptics), in terms of the risk of CRI or, secondarily, of PI48.
- -
The use of mupirocin for the care of the exit site reduces the risk of catheter infection and, probably, of PI due to Staphylococcus aureus. Although scantly orthodox, this strategy is supported by important evidence, including randomized trials and meta-analyses95. The percentage reduction of the risk of PI due to this microorganism is about 70%, versus 41% in the case of PI in general127. However, the optimum administration regimen has not been established to date — though there are data suggesting that intermittent administration involves a greater risk of bacterial resistances.
- -
No specific recommendation has been made for patients on PD and colonized by methicillin-resistant Staphylococcus aureus, due to the limited body of information on the subject128. Although a meta-analysis129 documented a relatively low prevalence in these patients (1.3% versus 7.1% in hemodialysis; p = 0.01), the association between this condition and the risk of PI could not be analyzed. The evidence on the association between resistance to methicillin and mupirocin is inconsistent91.
- -
Gentamycin cream applied to the exit site may be an alternative to mupirocin, with potentially greater efficacy in preventing CRI and PI due to gramnegative organisms92 — though its superiority has not been demonstrated in other studies130, and the risk of the development of resistances is potentially greater than in the case of mupirocin91,131.
- -
Other topical care options such as ciprofloxacin132, Melaleuca alternifolia (tea tree) oil, antibacterial honey133 or antibiotic associations134 have not demonstrated superiority, and indeed have evidenced certain inconveniences, when compared with the alternatives described above. Older strategies such as oral rifampicin likewise do not appear to find a place in the current protocols. A more recent randomized trial has highlighted the usefulness of polyhexamide in this context, though the control group was treated with saline solution and povidone iodine135.
- -
Based on common sense, the importance of early treatment for CRI is emphasized as a means to prevent progression towards PI.
No further relevant evidence has been forthcoming following publication of the ISPD guides. Mention can be made of some confirmatory meta-analyses95,96 and of a randomized trial that has shown the alternation of mupirocin and gentamycin to be associated to higher PI rates due to gramnegative organisms and fungi than monotherapy with gentamycin136. On the other hand, a meta-analysis137 has confirmed the pre-existing impression that the risk of PI is similar after local care of the catheter orifice with gentamycin or mupirocin (relative risk 1.03, 95%CI 0.72–1.47).
This Committee supports the recommendations of the ISPD, but wishes to establish some additional considerations:
- -
We consider that daily monitoring and care of the exit site of the catheter is more advisable than a minimum frequency of twice a week, since it allows closer control and greater quality care of the orifice. Daily care should be recommended for all patients.
- -
Although common sense suggests the convenience of identifying nasal and peri-catheter Staphylococcus aureus carriers and to treat only these patients with mupirocin, there is no evidence that this strategy is superior to direct treatment of all patients, in terms of both the prevention of infections and the development of resistances. It must be underscored that early or late recolonization is the norm following the treatment of carriers93,138. Moreover, identification of the carriers through periodic smear testing generates considerable costs that may reduce the cost-efficiency of the treatment. On presenting this issue to the panel of experts, no uniform criterion was observed, though most of the panel (57%) advocated the monitoring of recolonization as a guide for indicating treatment.
- -
We consider that, provided the necessary means are available, the sensitivity of Staphylococcus aureus strains to mupirocin should be monitored. However, it should be noted that so-called low-grade resistance, which is much more common, does not usually entail clinical risks and is not predictive of high-grade resistance (which does have clinical repercussions), since the latter manifests through different pathogenic mechanisms91.
Quality of evidence: Low
Synthesis of the evidence
This question has been addressed in a succinct manner by the ISPD guides of 201614. It is therefore advisable to take as reference the guide of 201725 on the prevention of CRI. Nevertheless, the recommendations or comments of both guides are mainly based on opinions (exit site care at least twice a week, immobilization of the catheter, short cycles of local or systemic antibiotic treatment for damaged exit sites, use of isolation systems for patients that practice swimming), while other relevant questions (dressing versus no dressing, indicated type of antiseptic / disinfectant, convenience of catheter tunneling following insertion) cannot be answered due to a lack of evidence or to poor quality evidence. Likewise, no evidence following publication of the mentioned guides has been forthcoming, since the only relevant study comparing dressing versus no dressing of the exit site124 was carried out in an environment very different to the Spanish setting.
The use of topical antibacterials applied to the exit site of the catheter for the prevention of PI is addressed in Structured question P5.
From evidence to recommendation
This Committee supports the conclusions of the ISPD guides of 201614 and 2017 25, with some essentially opinion-based nuances and considerations.
Recommendations
- -
We recommend regular monitoring and care of the exit site of the peritoneal catheter, if possible on a daily basis, as an indirect way to prevent PI (1C)
- -
We recommend early treatment of CRI, in order to prevent progression to PI (1C)
- -
We suggest the use of immobilization systems to avoid repeated traction upon the peritoneal catheter, at least during the post-implantation healing phase (2C)
- -
We suggest that each center should decide the type of soap or topical antiseptic for care of the catheter orifice, in accordance with the conditions of each patient (2C)
- -
We suggest that each center should decide the type of care or dressing of the catheter exit site (open or closed), in accordance with the conditions of each patient, as there is no evidence generally supporting any specific type for preventing CRI and PI (2C)
Quality of evidence: High
Synthesis of the evidence
This question has been extensively addressed by the ISPD guides of 2016 on PI14 and the guide of 201725 on the prevention of CRI. The available evidence supports the use of topical antibacterials for the prevention of infections in PD. The benefit is more patent in relation to CRI, but also applies to PI. Mupirocin is the reference antibacterial, though gentamycin probably yields similar efficacy, and may offer advantages in centers with a high incidence of infections produced by gramnegative microorganisms. Of note is the lack of data on the convenience or not of diagnosing carriers of Staphylococcus aureus, or on which treatment regimens (only peri-catheter or also nasal, continuous versus intermittent administration) are best. Since the publication of the ISPD guides, little additional information has been forthcoming, though there have been some confirmatory meta-analyses. A randomized study136 suggests that the strategy of alternating mupirocin and gentamycin may be counterproductive.
From evidence to recommendation
This Committee supports the recommendations ISPD, with some nuances and considerations.
Recommendations
- -
We recommend the administration of topical mupirocin or gentamycin during care of the exit site of the peritoneal catheter, as a way to reduce the risk of CRI and PI (1B)
- -
We suggest the convenience of periodic monitoring of the possible appearance of bacterial resistances to mupirocin or gentamycin among the bacterial flora (particularly Staphylococcus aureus) colonizing the nasal passages and the peri-catheter area (2B)
- -
This Committee provides no recommendation on the convenience of daily versus intermittent administration of the mentioned antimicrobials (ungraded)
- -
This Committee considers that the evidence does not warrant the treatment of nasal carriers of Staphylococcus aureus to reduce the direct risk of PI, though treatment may prove to be of benefit through reduction of the risk of CRI (2B)
The ISPD guides of 2016 does not go much into detail regarding asepsis during peritoneal dialysis exchange, and largely makes reference to previous documents on the subject11. There is general agreement among the professionals dedicated to PD on the advisability of correct hand hygiene during the exchange procedure. It is also recognized that such hygiene is often gradually neglected by patients over time — this representing a motivation for home visiting and retraining programs78,85,86. Although the different elements of adequate hand hygiene in dialysis exchange have been reviewed in depth139, there is very little controlled information on the specific impact of different factors (timing and duration of hygiene, appropriate type of soap, hand drying method, role of antiseptics or the wearing of gloves, etc.) upon the risk of PI. Some of the points to be remembered are:
- -
Washing should last at least 15 s140,141, though some groups extend this to a minimum of 30 s.
- -
The risk of contamination and transmission increases when the hands are not dried142, and it has been seen in patients on PD that wet hands hold up to 100 times more bacteria than hands that have been towel dried for 15 s143,144. The ideal drying method (towel, paper towel or hot air) has not been established. When a towel is used, drying ideally should last about 15 s, and this time should be extended to 45 s when a hot air stream is used, for drying of the hands.
- -
Failure to remove rings when washing significantly reduces the efficacy of hand hygiene145.
- -
Long native145 as well as artificial nails146 reduce washing efficacy and thus should be avoided as far as possible.
- -
The usual soaps do not contain antimicrobial agents. They are able to eliminate dirt, but different studies show that the residual bacterial count is substantial147.
- -
There are different types of antibacterial soaps. Some of them (such as those based on triclosan) are associated to significant bacterial resistance rates139.
- -
It is strongly advisable to use alcoholic disinfectants (usually based on ethanol, isopropanol or n-propanol) after hand washing. These products offer antibacterial activity against grampositive and gramnegative microorganisms, including methicillin-resistant Staphylococcus aureus. A number of studies have shown that gels and solutions based on water-alcohol formulations improve the results of washing with water and soap148–151. In patients on PD, it has been reported that the sequence of rubbing the hands with 70% ethanol after washing with soap is more effective than either measure applied separately151. Although disinfection with water-alcohol formulations is an adequate alternative in cases where the patient lacks adequate elements for hand washing, it is the majority opinion of the panel of experts (64%) that the use of these products ideally should follow correct hand washing152.
- -
The wearing of sterile gloves is an option in patients with problems for correct disinfection of the hands (including different allergies or skin diseases), though the results of this practice have not been established.
No information is available on how the conditions of the zone of exchange affect the risk of peritonitis. Traditionally, an exclusively dedicated, clean and well ventilated and illuminated room with access to running water has been considered ideal. However, many patients perform dialysis exchange in bedrooms or other shared zones, with no evidence that this circumstance increases the risk of infection.
The ISPD guides of 2016 indicates that the wearing of a facemask during the exchange is optional14. This decision is due to the fact that the evidence on the subject is scarce, weak and contradictory153,154. A related issue is the influence of dental and periodontal health upon the risk of PI. Patients on dialysis have higher periodontal disease rates than the general population, and this complication has been associated to the presence of systemic inflammatory markers155. A retrospective study involving 75 patients on PD156 has evidenced that those subjects who maintain higher standards of oral hygiene have significantly lower PI rates.
Domestic pets are a recognized potential cause of PI157–159, with important morbidity and mortality, as evidenced by some studies157. A recent review159 suggests a number of contamination routes: pets playing or resting on the cycler, resulting in contamination, or biting of the catheter or contamination of the hands and oropharynx of the patient. It seems reasonable to establish barriers to prevent pets from accessing the zone in which dialysis exchange is carried out, and to maximize the measures of hygiene on the part of the patient.
Quality of evidence: Low
synthesis of the evidence
This question has been addressed in a succinct manner by the ISPD guides of 201614, which makes reference to other ISPD documents11. In general, there is no evidence allowing firm recommendations to be made. Likewise, there have been no recent studies offering new information.
From evidence to recommendation
The recent evidence does not allow modification of the current points of view of the ISPD11,14, except as regards some minor aspects.
Recommendations
- -
We recommend that PD always should be performed in a clean environment, preferably with nearby access to running water (1C)
- -
We recommend correct hygiene before performing PD, with washing of the hands using common or antibacterial soap, followed by rubbing of the hands with a water-alcohol solution (1C)
- -
We recommend avoiding physical contact between domestic pets and the materials used for PD. In addition, patients should maximize asepsis and disinfection if coming into contact with pets (1C)
- -
This Committee suggests the convenience of wearing a facemask for exchange, particularly in patients with poor dental and periodontal health (2C)
The ISPD guides of 2016 recommends the use of double bag (“flush before fill”) systems as the best connection option for exchanges in PD. This technique is warranted by the experience of three decades and by conclusive meta-analyses47–49. The evidence supporting the use of double bag systems versus double-connection Y-set systems is not so conclusive, but is nevertheless probably sufficient. This latter comparison is of scant relevance in Spain, since double-connection systems are little used in this country.
Since 2016 there has been no new information to modify the criteria described above. Some devices have returned to the concept of ultraviolet (UV) disinfection161, though without evidence supporting the results. On the other hand, a recent randomized trial162 has compared the results of an incremental PD regimen (3 exchanges) versus a conventional regimen involving four exchanges in 139 incident patients on PD. A fewer number of connections was associated to a lesser risk of PI, though statistical significance was not reached.
Quality of evidence: High
Synthesis of the evidence
This is one of the few issues with near total agreement among the professionals dedicated to PD. The ISPD guides of 201614 provides a clear indication that has not been modified by subsequent evidence.
From evidence to recommendation
The recent evidence does not allow modification of the current points of view of the ISPD14.
Recommendations
- -
We recommend the use of systems with disconnection and purging before filling as the option of choice for connection in PD (1A)
- -
We suggest that double bag systems are preferable to the double-connection Y-set systems (little used in Spain) (2B)
In its day, the fewer number and greater concentration in time of the dialysis connections raised considerable interest in the possibility that automated peritoneal dialysis (APD) might be able to reduce the PI rates to below those associated with manual PD. The first randomized studies appeared to confirm this possibility163. However, in the first decade of this century, large observational registries documented similar PI rates with both modalities164–166. This situation led the ISPD guides of 2016 to not consider the indication of APD for reducing the risk of PI14. The reason for the discrepancy with respect to the older studies could be related to differences in the connection systems (use of threaded connectors versus pin systems, use of Y-set systems in manual PD, etc.).
Following publication of the guide of 2016, a new observational study was carried out by the BrazPD working group167, which once again evidenced similar risk with manual and automated PD.
Quality of evidence: Moderate
Synthesis of the evidence
The only randomized trials are old and with a low level of evidence163. The ISPD guides of 2016 is based on the results of different registries, which on the other hand coincide considerably in their results. Posteriorly, a new multicenter study167 has ratified the apparent absence of effect of the modality of PD upon the risk of PI, provided similar connection systems are compared.
From evidence to recommendation
The recent evidence does not allow modification of the current points of view of the ISPD14.
Recommendations
- -
We recommend that the prevention of PI should not be included among the indications for treatment with APD (1B)
The use of so-called biocompatible solutions has become consolidated in the last decade, based on their real or purported benefits in terms of preservation of the peritoneal membrane, residual kidney function, and peritoneal defense mechanisms. With regard to this latter aspect, a significant number of clinical trials have been published, with contradictory results51,168–176. In view of this scenario, and based on meta-analyses that report equivocal results and considerable heterogeneity50,52,177, the ISPD guides of 2016 does not recommend these solutions as an instrument for the prevention of PI in PD14.
Since the publication of the ISPD guides of 2016, a new clinical trial randomized 78 prevalent patients on PD to biocompatible or classical solutions (buffered with lactate), with a follow-up of two years178. The incidence of peritonitis was found to be lower in the biocompatible solution group. A recent meta-analysis (that does not include the previously mentioned study of Farhat)179 essentially confirmed the impossibility of establishing a firm recommendation on this subject.
The ISPD guides of 2016 issues no statement on the effect of peritoneal glucose or icodextrin load upon the incidence of PI. A recent analysis of the BalANZ trial180 identified no correlation between peritoneal glucose load (stratified by median values) and the risk of infection, the type of microorganism isolated, or the clinical course. With regard to icodextrin, data from meta-analyses179,181 evidence no apparent association to the risk of PI.
Quality of evidence: High
Synthesis of the evidence
Despite a significant number of randomized trials, complemented by meta-analyses50,52,179, the evidence for answering this question is contradictory. This is largely due to the great heterogeneity of the existing studies, many of which are characterized by low statistical power and insufficient patient follow-up. The trial published by Farhat et al.178 supports a beneficial effect of biocompatible solutions, but lacks the level of evidence needed to modify the general perception on this issue.
From evidence to recommendation
This Committee supports the recommendations of the ISPD guides of 201614.
Recommendations
- -
We suggest that the use biocompatible solutions has no confirmed effect upon the risk of PI (2A)
The frequency of disruption of asepsis in the course of treatment with PD is not clear though, in view of the many connections which the patient performs each year, it can be presumed that such disruptions are not rare. A previous study offered a series of guidelines (ungraded opinions) to deal with these situations, including40:
- -
The patient should be instructed during training on the potential risks associated with failure to adopt measures of protection after contamination, including immediate reporting of the event to the reference center.
- -
Sampling for peritoneal effluent culture (optional).
- -
Prophylactic coverage against grampositive and gramnegative microorganisms.
- -
Any positive isolation should be dealt with as PI, even in the presence of a clear effluent, but should not be counted as such, due to the lack of an inflammatory response. If the isolated organism corresponds to a common microorganism of the skin, a confirmatory sample should be obtained before starting antibiotherapy.
- -
If contamination through contact (including direct contact or the detection of bag rupture) was not followed by infusion, replacement of the transference line may suffice.
- -
In the event of any doubt as to whether infusion occurred, assume that it did occur.
A retrospective study covering a 15-year period182 compiled 548 episodes of contamination. When the latter was only related to inappropriate contacts, with the transference system closed and without the infusion of dialysate (“dry” contamination), no PI episodes were recorded. In contrast, 5.6% of the so-called “wet” contaminations were followed by infection. Notoriously, the administration of antibiotic prophylaxis greatly reduced the risk of infection (to 0.5%). Although the design of this study resulted in a low level of evidence, its results directly support the data of Bender et al.40.
Since the introduction of the ISPD guides of 2016, no controlled data on the subject have been published, though the nomenclature on accidental contamination (“wet” versus “dry”) appears to have been accepted183. Likewise, no information is available on the type of antibiotics, the administration route or the duration of prophylaxis, in these cases.
Quality of evidence: Low
Synthesis of the evidence
The indicated approach to accidental contamination has not been analyzed by studies with a sufficient level of evidence. The recommendations are based on common sense and on a retrospective and uncontrolled analysis, though with clear results that warrant prophylaxis for situations of “wet” contamination182.
From evidence to recommendation
This Committee supports the opinion of the ISPD guides of 201614. Although antibiotic coverage is considered to be advisable following “wet” contamination, the level of evidence is considered to be insufficient to establish a strong recommendation.
Recommendations
- -
We recommend that patients should be specifically instructed during training in PD on how to act in the event of accidental disruption of asepsis (1C)
- -
We suggest that “wet” contamination or contamination of an uncertain nature should be dealt with by replacing the connection line and administering antibiotic coverage via the oral or parenteral (intravenous or intraperitoneal) route, and covering both grampositive and gramnegative organisms, with a minimum duration of two days (2C)
- -
We recommend that “dry” contamination at least should dealt with by replacing the connection line (2D)
Invasive gastrointestinal procedures (including endoscopies and biliary tract interventions) and gynecological techniques manipulate areas located close to the peritoneal cavity; consequently, they may constitute a source of contamination and PI. Although the available information is scarce and with a low level of evidence, the ISPD guides of 2016 contemplates certain aspects that may help to establish a series of guiding criteria14:
- -
Biliary tract, lower gastrointestinal and gynecological procedures pose a defined risk of secondary PI, though this risk has not been quantified.
- -
The administration of antibiotic prophylaxis could reduce the risk of PI in this context184,185.
- -
In contrast, uncomplicated upper gastrointestinal procedures do not appear to imply a risk of PI185.
- -
There is no evidence regarding a specific risk of PI following urological instrumentations, though most centers administer antibiotic prophylaxis based on the potential risk of bacteremia. There is no evidence supporting the routine administration of antibiotic prophylaxis in the case of bladder catheterization.
- -
No optimum antibiotic prophylaxis regimen has been established, though it seems reasonable for coverage to include enterobacteria and intestinal anaerobes. The coverage of yeasts does not appear to be indicated on a systematic basis, but may be considered in situations of risk.
- -
Likewise, there is no evidence supporting the tendency of some centers to void the peritoneal cavity for the procedure.
Since the publication of the guide of 2016, some further evidence of interest has emerged. A randomized trial recorded no apparent benefit of antibiotic prophylaxis with ceftazidime in patients on PD subjected to colonoscopy186. More recently187, a large retrospective observational study recorded secondary PI in 3.8% of the colonoscopic procedures performed in patients on PD. No patient receiving antibiotic prophylaxis suffered this complication. Notoriously, the incidence of PI in patients subjected to polypectomy was three times higher than among patients that did not undergo this procedure. In another less weighted study188, 26 gynecological procedures were complicated by 7 episodes of PI. The risk of infection was significantly lower among the patients that received antibiotic prophylaxis — though the sample size limited the reliability of the results obtained.
A recent article has re-evaluated this issue, advocating antibiotic prophylaxis also before upper gastrointestinal tract endoscopies, as well as voiding of the peritoneal cavity, in order to improve the peritoneal defenses183. However, this was an opinion document that did not in itself contribute evidence.
Transient bacteremia is common after dental procedures and can lead to PI. The high prevalence of periodontal disease among patients on PD may increase this risk155. It seems reasonable to administer antibiotic prophylaxis at least in procedures associated with a greater bacteremia risk (extractions, periodontal cleaning, endodontic treatments, dental implants), but the possible benefits of this strategy have not been clearly established. Overall, this suggestion is supported by 92% of the members of the panel of experts.
Quality of evidence: Low
Synthesis of the evidence
There is no evidence to reliably answer this question. The incidence of PI after colonoscopy is 6−25% when prophylaxis is not provided, and 0−6.5% when antibiotic prophylaxis is prescribed. The ISPD guides of 201111 and 201614 recommend voiding the abdomen and the administration of antibiotic prophylaxis. Two recent studies, one involving a randomized design but with low statistical power185, and another observational study with a large number of patients187, have yielded contradictory results. In relation to gynecological explorations, the evidence is still scarce, though antibiotic prophylaxis seems reasonable, in the same way as in bacteremic dental procedures. A small observational study188 has associated antibiotic prophylaxis with a decrease in the risk of PI following gynecological explorations. No information is available on the prophylactic regimens best suited to each type of procedure.
From evidence to recommendation
This Committee supports the opinion of the ISPD guides of 201111 and 201614.
Recommendations
- -
We recommend that patients on PD scheduled for invasive biliary, lower gastrointestinal or gynecological procedures should receive appropriate antibiotic prophylaxis [1C]
- -
We suggest that the provided antibiotic prophylaxis should be active against common enterobacteria and intestinal anaerobes (2C)
- -
We suggest that antibiotic prophylaxis is not necessary in endoscopic upper gastrointestinal tract explorations (2C)
- -
We suggest that the available information does not allow us to establish the convenience of voiding the peritoneal cavity for the above mentioned procedures (2D)
- -
We suggest that routine antibiotic prophylaxis be considered for dental procedures, especially those with a greater bacteremic potential (ungraded)
Although PI due to fungal microorganisms is infrequent (1–14% of all cases of PI), it constitutes one of the most feared complications of PD, due to its high mortality rate and associated failures of the technique189–193. The fact that between 56–89% of all such episodes appear after bacterial infection (whether peritoneal or otherwise) treated with antibiotics189,190,194 has generated recurrent interest over the last 30 years regarding the convenience of prescribing antifungal prophylaxis in these clinical contexts. Although multiple studies with low levels of evidence have been published, randomized trials195,196 and meta-analyses119,120 with positive outcomes have served as the basis of the ISPD guides of 2016 for supporting this measure. The guide does not include suggestions on other concrete clinical contexts, or on which antifungals are to be preferred or the duration of prophylaxis in these cases — though it does appear to acknowledge a certain advantage of azole drugs over nystatin14. Eighty-four percent of the members of the panel of experts share this same opinion.
No new evidence of interest has appeared since the publication of the ISPD guide. A descriptive observational study193 has not contributed anything new. A meta-analysis96 in turn has confirmed the previous impressions, with no further evidence. Two recent surveys3,197 describe great variability in the adoption of this practice in different parts of the world, ranging from 0 to 70%, as well as variable criteria for the use of such treatment. There is a notorious lack of information as to which antibiotic treatments should combine antifungal prophylaxis.
Quality of evidence: Moderate
Synthesis of the evidence
The recommendation of the ISPD guide is based on two small, randomized trials with heterogeneous designs195,196. The relevance of the complication has probably impulsed the recommendation, the potential benefits being considered to far outweigh the risks. Since the ISPD guides of 2016 there has been no new information to modify this criterion.
From evidence to recommendation
This Committee supports the opinion of the ISPD guides of 201614.
Recommendations
- -
We recommend that patients on PD administered oral or parenteral antibiotic treatment should receive oral antifungal prophylaxis during the treatment period (1B)
- -
We suggest that azoles may be the most appropriate choice for this purpose, though nystatin may be an adequate alternative (2C)
In addition to the measures commented up to this point, many other strategies for the prevention of PI have been proposed. In general, these approaches are based on observational studies that detect associations between a given factor and the risk of infection (Table D1)5,177,198–202. As is logical, little can be done about the non-modifiable risk factors, apart from disadvising PD in those patients who present such factors. Correct patient selection is therefore of great theoretical importance for reducing the posterior incidence of PI. However, with the exception of obvious conditions (e.g., patients with active inflammatory bowel disease or a history of diverticulitis), it is very difficult to predict whether a concrete individual will suffer frequent PI episodes, and the existing risk models are generally weak and inconsistent36,165,203. This Committee considers that it is more logical to adopt an individualized approach and to focus on correct training than to exclude patients simply because they belong to a certain risk group, where risk at an individual level is hard to establish.
In principle, focusing attention on the modifiable risk factors177 seems more promising, though in practice it faces important difficulties. The association of some of these factors (e.g., smoking) to the risk of PI is controversial. There is also no evidence that the correction of other factors (such as obesity or depression) is able to reduce the risk of infection. However, in other cases, the available evidence does deserve some consideration.
The prevention of enteric PI is a challenge for PD units, in view its high frequency and clinical aggressivity. Many of these infections originate from pre-existing gastrointestinal disorders. For years it has been speculated that factors which weaken the antimicrobial intestinal barrier can increase the risk of enteric PI. In this regard, the ISPD guides of 2016 briefly comments on the convenience of avoiding abnormal peristalsis — whether reduced (constipation, intestinal paresis) or accelerated (gastroenteritis) — though stressing the weakness of the evidence14. A relationship has been postulated between hypokalemia and infection due to enteric organisms204,205, though here again there is no direct evidence to suggest that correction of the disorder reduces the risk of this complication. A single study, with a low level of evidence206, has related the regular use of laxatives to a lesser risk of PI.
At the time of drafting of the ISPD guides of 2016, two observational studies207,208 related low 25-OH-vitamin D3 levels to an increased risk of PI.
A number of studies of interest have appeared since the publication of the guide of 2016, though with limited levels of evidence. A meta-analysis of 6 observational studies (totaling 3613 patients) concluded that the evidence relating hypokalemia to the risk of PI is very inconsistent209. On the other hand, two retrospective observational studies210,211 have detected an association between treatment with gastric acid secretion inhibitors and the risk of PI. In the first of these studies, the observed association was limited to infections caused by enteric microorganisms, and it proved much more consistent with H2 receptor antagonists than with proton pump inhibitors210. Lastly, two observational studies212,213 have related the existence of sustained overhydration to a risk of PI due to enteric organisms in patients on PD. In the more statistically potent of these studies211, the relationship was apparently consistent, though there was evidence of a confounding factor in the form of the presence of malnutrition.
An observational study214 has related plasma vitamin D levels to the risk of PI. Notoriously, the association did not depend on the administration of oral vitamin D supplements. A more recent meta-analysis215 including 5 studies with patients on PD, 11 studies with patients on hemodialysis, and one study with both types of treatment, demonstrated a lowered general risk of infection among the patients with normal or elevated vitamin D levels. In addition, the patients administered oral vitamin D supplements likewise showed a reduced risk of this type of complication. The significance of this meta-analyses in relation to the topic of this document is limited, due to the heterogeneity of the analyzed studies.
Quality of evidence: Low
Synthesis of the evidence
The ISPD guides of 2016 comments only briefly on this subject and offers no recommendations. Although a recent meta-analysis215 describes a decrease in the risk of infection in the presence of medium to high levels of vitamin D and/or the administration of oral vitamin D supplements, the applicability of these findings in the specific field of PD is doubtful.
From evidence to recommendation
This Committee adds a suggestion on this issue in relation to the absence of recommendations in the ISPD guides of 201614.
Recommendations
- -
We suggest the convenience of maintaining adequate patient plasma vitamin D levels (with the prescription of supplements if needed), in order to reduce the general risk of infection in patients subjected to PD (2D).
Peritoneal infection (PI) is to be managed from the time of diagnosis as a potentially serious complication of PD, for two main reasons:
- a)
As has been commented in the introduction to this Guide, PI can have undesirable clinical consequences and repercussions: adverse effects of diagnostic and therapeutic procedures, a possible need for hospital admission, or failure of the technique30–35 and – much more importantly – a low but not negligible direct mortality risk5,36,37.
- b)
The initial clinical presentation of PI is not a reliable indicator of its course or prognosis. Serious infections (including enteric and fungal infections) initially may appear indolent, while others that will subsequently evolve without consequences may initially manifest with an alarming inflammatory response (intense pain, impaired general condition, marked turbidity of the effluent). Systematically regarding PI as a potentially serious complication is the most conservative and sensible strategy.
The general principles of treatment are described in Table T1. A correctly trained patient should be aware of the importance of seeking help immediately in the event of signs or symptoms of PI. Although there is no direct evidence that immediate treatment improves upon the outcomes of treatment started with one or more days of delay, the general principles of the management of infectious disorders suggest that immediate care does improve the outcomes, and it is accepted that the number of days of peritoneal inflammation influences the chances of preserving the peritoneal membrane and the PD technique itself216. For this same reason, the induction of rapid remission of the infection should be a fundamental objective.
General principles for the initial treatment of peritoneal infection.
| The diagnosis and start of treatment should be as early as possible after PI signs/symptoms onset |
| Treatment should not be started without adequate prior collection of bacteriological samples |
| Initial (empirical) treatment of PI should adequately cover grampositive and gramnegative bacteria |
| The choice of initial (empirical) antibiotherapy should take into account the local microbiota and the usual antibiotic susceptibility patterns in the center, with due observation of the above principle |
| Residual kidney function is an important factor both for treatment selection (avoid nephrotoxic antibiotics) and for defining the antibiotic doses |
| Adequately plan follow-up (admission, time to review), taking into account the clinical condition of the patient, response-limiting factors (immune suppression, simultaneous tunnel infection, etc.) and the risk of inadequate home treatment (poor adherence, limited capacity, etc.) |
Initial empirical antibacterial treatment cannot cover the full microbial spectrum, but should at least cover the main infections, including those caused by grampositive cocci and gramnegative bacilli. Empirical coverage should be adjusted to the usual microbiota prevalent in each Unit. The local incidences of infections caused by Staphylococcus aureus and non-fermenting gramnegative bacilli (particularly Pseudomonas spp.) are the factors that most commonly modify the initial treatment protocols.
The dosing of antibiotherapy should be at the upper limit of the desirable range for the patient, considering his or her residual kidney function and other factors that can modify the efficacy and safety profile (age, liver disease or possible drug interactions, among others). Many centers use antibiotic doses above the recommended doses in the first days of treatment, in order to reach appropriate intraperitoneal and systemic drug levels quickly, while the causal microorganism remains to be identified. There is no evidence that this strategy improves the outcomes of treatment, however. A recent uncontrolled observational study217 recorded a greater risk of treatment failure in patients with residual kidney function, which the authors attributed to the risk of underdosing in this subgroup of individuals. Although the protocol of the mentioned study included an aminoglycoside in oliguric patients and ceftazidime in patients with residual kidney function – which also could have influenced the results obtained – the findings obtained should draw attention to the possible negative consequences of antibiotic underdosing in PI.
The initial diagnosis of PI includes assessment of the risk profile of the patient and of the repercussions of the infection upon his or her general condition. Consideration is also required of factors that can limit the response to antibiotherapy as sole treatment strategy, such as the presence of wall abscesses or signs of background abdominal disease. These factors influence the ulterior management decisions, particularly as regards whether treatment should be provided on an ambulatory basis or following admission to hospital. In general, and based on clinical criteria, approximately 80% of all cases of PI could be managed on an outpatient basis59. However, this decision is also influenced by factors such as the social setting of the patient, the patient skills, the distance to the center, the infrastructure of each Unit, or its training protocols (which do not always include instruction on intraperitoneal drug administration). These factors cause the actual admission rates due to PI to vary greatly between centers, with figures often above 50%5.
Initial therapyChoice of antimicrobialsAdequate knowledge of the local microbiota conditions the initial treatment of PI. In its defect, the ISPD14 suggests administering vancomycin or a first-generation cephalosporin to cover grampositive microorganisms, and an aminoglycoside or third-generation cephalosporin to cover gramnegative microorganisms. However, some aspects need to be considered:
- -
For different reasons (Table T2), multiple initial treatment protocols have been developed for PI in PD. The scarce available comparative evidence between different strategies, together with local factors, limit the scope of any attempt to establish general recommendations. The high prevalence of allergy (real or purported) to betalactam antibiotics – which are the basis of most of the treatment regimens – often makes it necessary to have secondary protocols. In these cases, there are effective alternatives for the treatment of infections due to both grampositive (vancomycin, teicoplanin, daptomycin or linezolid) and gramnegative microorganisms (aztreonam, aminoglycosides, quinolones).
Table T2.Causes of variability in the antibiotic treatment protocols for peritoneal infection.
Diagnostic efficiency of the Unit (quality of Microbiology) Local etiological spectrum Antibiotic susceptibility of the local microbiota Residual kidney function Clinical context (limiting factors or factors that complicate the response) Antibiotic allergies or intolerance Diversity of PD procedures (automated PD and others) Antibiotic versatility regarding administration routes and dosing intervals Restrictive versus proactive vision of the use of new antibiotics Economic resources and local availability of antibiotics Scarcity of controlled, quality data (evidence) - -
Although short cycles of aminoglycosides are agreed to be scantly toxic, the natural tendency, provided the microorganism is sensitive, is to continue with the initial treatment regimen. For this reason, it seems reasonable to limit the use of this drug family in patients with residual kidney function. In contrast, vancomycin offers a very convenient empirical therapeutic profile that compensates its potential toxicity, and the incidence of resistances is relatively low in Spain.
- -
Gram staining is of limited sensitivity. Furthermore, even if grampositive or gramnegative bacteria are detected, this does not rule out the possible growth in culture of other microorganisms. In any case, gram staining does help to define the initial antibacterial management strategy. Its greatest usefulness is in the early detection of the presence of yeasts58, which would radically modify the management approach.
A review of the literature posterior to the guide of 2016 has only revealed two relevant contributions. In a small single-center randomized trial, Xu et al.218 showed that oral moxifloxacin or intraperitoneal ceftazidime as an adjuvant to intraperitoneal vancomycin offers equivalent resolution rates (78% versus 80%; p = 0.80). In a more recent multicenter randomized trial219, the treatment success rate (82.6% versus 81.1%; p = 0.4), as well as other secondary outcome variables, were found to be very similar on comparing intraperitoneal cefepime versus cefazolin with ceftazidime via the intraperitoneal route. According to the authors, this demonstrated the efficacy of cefepime monotherapy versus the conventional protocols.
The main advantages and inconveniences of the different antibiotic groups as initial empirical treatment for PI are reported in Table T3. This table clearly evidences why vancomycin and cephalosporins are the preferred choices. Carbapenems and daptomycin are excellent alternatives for covering gramnegative and grampositive microorganisms, though the cumulative experience in this case is more limited; as a result, many centers regard them as options held in reserve, in view of the efficacy still shown by the more traditional drug associations, which remain the first choice.
Quality of evidence: Moderate
Synthesis of the evidence
The ISPD guides of 2016 provide an exhaustive review of the protocols regarding the empirical treatment of PI up to that year14. A review of the posterior literature only yielded two relevant contributions218,219, but which do not modify the previous scenario.
From evidence to recommendation
In the absence of new quality evidence, this Committee adheres to the general recommendation of the ISPD14, though places less emphasis on the utilization of aminoglycosides in patients with residual kidney function, given the potential renal toxicity of these drugs.
Recommendations
- -
We recommend that antibiotherapy be started as soon as possible when PI is suspected (1C)
- -
We recommend adequate coverage of grampositive and gramnegative microorganisms as part of the initial PI treatment protocol, in all cases (1B)
- -
We recommend adjusting the empirical treatment protocol for PI to the local epidemiology (1C)
- -
In the case of centers without sufficient experience of their own, we recommend vancomycin or a first-generation cephalosporin to cover grampositive microorganisms, and a third-generation cephalosporin or an aminoglycoside to cover gramnegative microorganisms (1B)
- -
We suggest limiting the use of aminoglycosides in patients with PI and significant residual kidney function (2C)
- -
We suggest the start of treatment for PI using antibiotic doses at the upper limit of the range recommended by the Summary of Product Characteristics, taking into account the residual kidney function of the patient (2C)
- -
We suggest the convenience of having alternative antibiotic protocols for patients with a history of allergies or intolerances to antibiotics, particularly betalactam drugs (2C)
Main advantages and inconveniences of the different antibiotic groups for the initial treatment of peritoneal infections in PD.
| Group | Advantages | Inconveniences |
|---|---|---|
| Glycopeptides |
|
|
| Penicillins and ampicillins |
|
|
| Cephalosporins and aztreonam |
|
|
| Carbapenems |
|
|
| Aminoglycosides |
|
|
| Quinolones |
|
|
| Daptomycin |
|
|
MRSA: methicillin-resistant Staphylococcus aureus; ESBL: extended-spectrum betalactamase.
In different sections, the ISPD guides14 addresses a series of accessory measures that may be of help in the initial management of PI. These measures include:
- -
The administration of analgesia proportional to the intensity of the pain of the patient. The addition of local anesthetics to the dialysate (e.g., 3−5 ml of 2% mepivacaine per bag) alleviates the pain, though oral or parenteral analgesia is usually required in most cases.
- -
Peritoneal lavage may prove useful in the presence of pain or important turbidity, to improve the mechanics of dialysis and mitigate the pain.
- -
The addition of heparin to the dialysate when drainage proves turbid or contains abundant fibrin can help avoid thrombus formation, which may worsen the mechanics of dialysis and aggravate the transient ultrafiltration deficit that characterizes the inflammatory phase of PI.
- -
A small, randomized trial, already contemplated by the ISPD guides of 2016220, showed that the use of icodextrin for the long exchange during the acute phase of PI allows the maintenance of adequate ultrafiltration in this phase. Since the study was unable to demonstrate clinical benefits with this measure, the indication should be individualized.
- -
Although there is some preliminary experience with catheter sealing using antimicrobials as an accessory measure for preventing relapses221–224, this practice has not been warranted by controlled studies, and therefore cannot be recommended at this time.
- -
Sealing with urokinase to eliminate the bacterial biofilm and thus prevent relapses was largely abandoned years ago, and there is no recent evidence to contradict this criterion225.
Following the introduction of the Guide of 2016, a small (40 episodes of peritonitis), single-center randomized trial analyzed the effect of peritoneal lavage (versus no lavage) upon the course of PI, with no apparent benefits being observed226.
Quality of evidence: Low
Synthesis of the evidence
This question has been conveniently addressed by the ISPD guides of 201614. In essence, the available evidence is scarce, and the different practices are endorsed by the opinions of experts. A recent study on the effect of peritoneal lavage (versus no lavage) upon the course of PI has demonstrated no apparent benefits226.
From evidence to recommendation
Here again, there is no evidence posterior to publication of the ISPD guides of 201614 capable of modifying the considerations of the latter.
Recommendations
- -
We recommend peritoneal lavage in the case of very turbid peritoneal drainage and/or intense abdominal pain, with the aim of providing pain relief and improving the mechanics of PD (1C)
- -
We suggest the addition of heparin to the dialysate in patients with a turbid effluent, in order to maintain good mechanics of PD (2C)
No relevant new data have been published capable of modifying the recommendations of the ISPD guides of 201614; the general considerations of the guide are therefore maintained. However, this Committee considers it pertinent to offer some comments.
The ISPD guides consider the intraperitoneal route (versus the intravenous route) to be the option of choice for the administration of antibiotherapy, with the exception of patients presenting signs of sepsis14. This Committee largely supports this recommendation (62% of the panel), though it considers the supporting evidence to be insufficient. Most of the antibiotics used for the treatment of PI are characterized by good peritoneal penetration following intravenous administration. Moreover, higher drug levels in the dialysate after intraperitoneal administration are not necessarily associated to greater tissue levels of the antibiotic. Likewise, there is no evidence of fewer adverse effects when the intraperitoneal route is used. The clinical evidence of the advantages of the intraperitoneal route with respect to the intravenous route is weak, since it is limited to some small clinical trials with vancomycin225. However, the intraperitoneal route is very convenient for ambulatory administration of the antibiotic, and this is perhaps the most convincing argument in its favor. Some additional considerations are also required:
- -
In hospitalized patients, the intravenous route does not usually pose administration problems, and may be more predictable in the presence of signs of serious infection (sepsis), as commented by the ISPD guide itself14.
- -
Intraperitoneal administration on an ambulatory basis has limitations. Many centers do not train their patients in this procedure (though this Committee unanimously considers such training to be recommendable), and patient manipulation of drugs destined for parenteral administration may generate some reservations.
- -
Most of the antibiotics used for the treatment of PI in PD are safe and remain stable in the dialysate for the necessary period of time14. Some centers provide their patients with bags of dialysate containing the antibiotic. This procedure is reasonably warranted by clinical practice, and there are continuous publications evidencing the stability of different antibiotics in PD solutions227–231. However, the clinical efficacy of protocols of this kind has not been evaluated on a controlled basis.
- -
Use of the ambulatory intravenous route is feasible in some patients, particularly in the case of antibiotics that are administered on a spaced basis (glycopeptides, aminoglycosides, ceftazidime).
- -
The continuous intraperitoneal administration of antibiotics generates uncertainties in some centers in relation to the management of patients on intermittent or semi-intermittent PD (especially APD) (see below).
The oral route has been regularly evaluated for the treatment of PI in PD, either as monotherapy or as part of combination treatment. Most of the studies are based on the use of quinolones or betalactams, with very little experience to date using linezolid. The oral route has some potential problems, including relatively slow penetration into the peritoneal cavity, unpredictable pharmacokinetics (many patients present reflex ileus in the presence of PI, and bioavailability may be affected by diet or other medications), and the potential risk of poor adherence in some patients – particularly when the symptoms start to subside. This Committee coincides with the considerations of the ISPD guides of 201614 in that the route is feasible, but of only secondary relevance.
Since the publication of the ISPD guides of 201614, there have been a relatively large number of in vitro studies or small pharmacokinetic studies seeking to confirm the stability of different antibiotics in PD solutions226–229, or the duration of the levels of different antibiotics following their intermittent oral or intraperitoneal administration231–234, though they have not modified the existing criteria on this topic. A single randomized trial218, already commented above, reported comparable therapeutic failure rates on adding oral moxifloxacin or intraperitoneal ceftazidime to intraperitoneal vancomycin as initial treatment for PI in PD. The trial only included 13 episodes of PI due to gramnegative organisms; the risk of beta error is therefore significant. A single-center, retrospective observational study234 reported similar efficacy with oral amoxicillin and intraperitoneal vancomycin for the treatment of enterococcal peritonitis in PD - with a level of evidence clearly insufficient to modify the regimens currently recommended for the management of these infections.
Quality of evidence: Low
Synthesis of the evidence
Since the publication of the ISPD guides of 201614, there have been only two studies of interest (218,232,235] warranting (with a low level of evidence) the oral route for the treatment of PI, though one of them218 added parenteral vancomycin in both arms of the trial.
From evidence to recommendation
In view of the lack of new quality evidence, this Committee supports the recommendations of the ISPD guides of 201614, with some comments related to the inconsistency of the available information.
Recommendations
- -
We recommend the intraperitoneal route as the preferred option for the treatment of PI on an ambulatory basis, provided the level of training of the patient is adequate (1B)
- -
In hospitalized patients, we likewise suggest the intraperitoneal route as the preferred option, though the intravenous route is also considered adequate – the decision being conditioned to the pharmacokinetic properties of the antibiotic employed and the clinical context (2B)
- -
We recommend that the treatment of PI should not be based on monotherapy via the oral route (1C)
This Committee generally agrees with the recommendations of the ISPD guides of 201614. The intermittent administration of aminoglycosides appears to be particularly advisable, in view of their efficacy-toxicity profile, and for preventing adaptive resistance phenomena236. The recommendation to also administer vancomycin on an intermittent basis is more questionable. We consider that there is no evidence that the intermittent or continuous administration of this antibiotic results in different clinical outcomes. The usual diurnal dwells in CAPD (about 4 h) do not guarantee full equilibrium of the administered dose237, though they do suffice in practical terms. Although vancomycin is potentially toxic, in the context of PI in PD, underdosing is likely to imply greater risks than overdosing. From the theoretical perspective, continuous administration would ensure greater stability of the drug levels, though neither regimen is able to replace the safety afforded by pharmacological monitoring — particularly in patients with significant kidney function, where underdosing is more probable217.
Although in general terms it is considered that betalactams improve their safety profile in continuous perfusion, this Committee assumes equivalence of the intermittent or continuous administration regimens of these antibacterials and similar drugs (carbapenems, aztreonam) for the treatment of PI in PD14. Given the need to administer more than one daily dose of many of these antibiotics, particularly in the presence of residual kidney function, we consider that continuous administration may be the best option for patients considered overall — though intermittent administration is appropriate for many of these compounds (e.g., cefazolin or meropenem)14,238.
Quality of evidence. Low
Synthesis of the evidence
There appears to be no new evidence to modify the considerations of the ISPD guides of 201614.
From evidence to recommendation
This Committee supports the recommendations of the ISPD guides of 201614, with some considerations as described below.
Recommendations
- -
We recommend that intraperitoneal aminoglycosides should be administered on an intermittent basis for the treatment of PI in PD (1B)
- -
We recommend that the intraperitoneal administration of vancomycin be prescribed on either a continuous or an intermittent basis (1B)
- -
We suggest that whenever possible, the plasma vancomycin and aminoglycoside levels should be monitored, especially in patients with residual kidney function (2B)
- -
We suggest that the intraperitoneal administration of betalactams and carbapenems be prescribed on either a continuous or an intermittent basis — the decision being conditioned by the dosing interval of each antibiotic (2C)
The versatility of APD is an advantage for patients, though it often gives rise to doubts when it comes to treating PI. This Committee agrees with the ISPD guides of 201614 in that the information available for establishing clear criteria is insufficient. Some considerations which this Committee wishes to establish are:
- -
The greater filtration capacity of APD versus CAPD may affect the pharmacokinetics of some antibiotics, including vancomycin237, generating a risk of underdosing in the former therapy. Consequently, if the plasma levels of the drug are not monitored, we should administer doses at the upper limit of the recommended range.
- -
The intermittent administration of antibiotics via the intravenous, intraperitoneal or oral route may follow the general criteria in patients on APD. Logically, if administration is via the intraperitoneal route, it should be carried out in a long exchange. The condition of dwelling for at least 6 h raised by the ISPD guides14 is very reasonable. In the case of cephalosporins, it has been shown that administration in short exchanges also generates adequate plasma levels239, but this option affords no advantages over administration in long exchanges. It is important to take into account that intermittent administration during long exchanges may imply relatively low levels of some antibiotics (such as vancomycin237 or the cephalosporins240) during the short exchange phases.
- -
In patients on intermittent nocturnal PD, we may consider introducing a diurnal exchange during the treatment period, in order to administer the antibiotic, if we have chosen the intraperitoneal route. This consideration is valid for both intermittent and continuous administration regimens.
- -
If we choose continuous intraperitoneal administration, the total dose of antibiotic should be divided between the diurnal and nocturnal exchanges. Some centers introduce a complementary diurnal exchange to ensure the intraperitoneal and plasma concentrations of faster absorbing and distribution antibiotics — though this practice is not supported by evidence. Other centers prefer temporary transference to CAPD, if the patient is admitted to hospital or knows the mechanics of the latter technique. This decision allows more homogeneous dosing, and can offer the professional greater subjective reinsurance as to how to administer the antibiotic, but it may worsen the ultrafiltration capacity in a critical moment, and there is no evidence that it improves the results of treatment.
- -
With regard to treatment via the oral route for PI in APD, the existing experience is still insufficient14.
- -
There is not enough evidence to assume a different response to treatment in PI among patients on CAPD or APD241,242.
No relevant evidence has been forthcoming regarding the management of PI in APD after publication of the ISPD guides of 201614. An already mentioned small and uncontrolled study239 showed that the administration of cefazolin or ceftazidime in short exchanges at decreasing concentrations (due to mixing with clean dialysate) affords adequate intraperitoneal levels of both antibiotics. Another study, with an even lower level of evidence232, showed that ciprofloxacin via the oral route at a dose of 750 mg every 12 h achieves appropriate intraperitoneal levels in patients on APD without PI. On the other hand, two exhaustive (but not systematic) reviews237,240 have underscored the possible convenience of continuous intraperitoneal administration of vancomycin and cephalosporins in order to avoid the presence of subtherapeutic levels in the nocturnal phase (short exchanges).
Quality of evidence: Low
Synthesis of the evidence
No relevant evidence has been forthcoming regarding the management of PI in APD after publication of the ISPD guides of 201614. Of interest are two non-systematic reviews237,240 suggesting the possible convenience of continuous intraperitoneal administration of vancomycin and cephalosporins in order to avoid the presence of subtherapeutic levels in the nocturnal phase (short exchanges).
From evidence to recommendation
This Committee supports the recommendations of the ISPD guides of 201614, with some minor considerations as described below.
Recommendations
- -
In patients on APD with PI treated using intraperitoneal antibiotics, we recommend the administration of vancomycin and betalactams on a continuous basis in order to avoid subtherapeutic drug levels in the dialysate during the short nocturnal dwells (1B)
- -
When choosing an intermittent intravenous, intraperitoneal or oral antibiotic administration regimen in patients on APD, we recommend the application of general criteria similar to those used in CAPD (1B)
- -
In patients on APD with PI, we recommend that in the case of intermittent intraperitoneal administration, the latter should be carried out in the course of a long exchange, with a dwell of at least 6 h (1B)
- -
In patients with a nocturnal intermittent PD regimen (dry day) to be treated via the intraperitoneal route, we suggest the temporary introduction of a diurnal exchange to facilitate administration (intermittent or continuous) of the antimicrobial treatment (2D)
- -
We suggest that the practice of transferring patients on APD to CAPD during the treatment of PI via the intraperitoneal route is not justified by therapeutic reasons, though it may be justified on a practical basis (2D)
Clinical monitoring is required from the start of empirical treatment (see section 13 of this guide). Some relevant considerations in this phase are:
- 1
Gram staining only modifies treatment in a radical way when polymicrobial flora and/or years are identified, though in the rest of positive cases it contributes to design the treatment strategy.
- 2
Bacterial cultures often yield results in under 24 h, though in some cases several days may be needed.
- 3
It is important to remember that asynchronic growth can occur, particularly in the case of enteric infections. Gramnegative microorganisms and especially anaerobes can exhibit delayed growth in culture. It is therefore very advisable to continue monitoring of the culture results beyond the initial isolation.
Once the causal microorganism/s has been identified, adjustment of antibiotherapy may be required. This Committee agrees with the ISPD guides of 201614 in that it is advisable to adjust the treatment spectrum to the isolates obtained. In certain cases, an antibiotic association should be maintained (Fig. T1). This strategy is common in PI caused by non-fermenting gramnegative bacilli (except perhaps PI due to Acinetobacter spp.243) and the majority of polymicrobial infections. The scenario in relation to enterococci is more dynamic, due to the growing experience gained with new antibiotics244, though ampicillin and glycopeptides remain the most solid basis of treatment.
The minimum duration of treatment for PI is two weeks, though the causal organism or the clinical presentation of the infection may advise a longer period14. The ISPD guides recommend the prolongation of treatment (21 days or more) in PI caused by certain microorganisms, essentially Staphylococcus aureus, Corynebacterium spp., Enterococcus spp., gramnegative bacteria, mixed flora and mycobacteria. In contrast, no such measures are advised based on the clinical course (clinical aggressivity, slow resolution) or complication risk factors (high-risk patients, catheter-related PI, treatment of relapses)14.
No study contributing solid evidence in relation to these issues has been found following publication of the ISPD guides of 2016. A recent systematic review245 underscores the scant attention paid to this field in recent years. The development of alternatives (including linezolid and especially daptomycin) for the treatment of PI due to enterococci244,246 is clearly of interest, though no controlled experiences are available for advising modification of the current recommendations. A multicenter observational study of 176 cases of monobacterial PI due to Acinetobacter spp. in the Anzdata survey243 only recorded antibiotic combinations in 13% of the cases, with similar outcomes in response to different forms of monotherapy. A recent retrospective, multicenter observational study of 153 cases of PI due to Pseudomonas spp. revealed no further benefit from adding a third antibiotic to the usual two-antibiotic regimen in the management of these infections247.
Quality of evidence: Moderate
Synthesis of the evidence
There has been no further evidence with respect to the ISPD guides of 201614 in relation to the duration of treatment for PI. This topic has not been addressed by the main studies on PI245.
From evidence to recommendation
This Committee agrees with the recommendations of the ISPD guides of 201614, with some additional considerations.
Recommendations
- -
We recommend a minimum duration of 14 days for the antibiotic treatment of PI in PD (1B)
- -
We suggest that it may be advisable to prolong treatment at least three weeks in PI due to Staphylococcus aureus, Corynebacterium spp., Enterococcus spp., gramnegative bacteria and mixed flora, with the participation of enteric organisms (2B)
- -
We suggest prolongation of the duration of treatment for at least one additional week when clinical criteria indicate an increased risk of a complicated course of PI (2C)
Quality of evidence: Low
Synthesis of the evidence
No study contributing solid evidence in relation to this issue has been found following publication of the ISPD guides of 2016. Some recent analyses have shown the viability of monotherapy in PI due to enterococci244,246, Acinetobacter spp.243 and Pseudomonas spp.247, but these are based on uncontrolled studies, and therefore do not modify the point of view of this Committee.
From evidence to recommendation
This Committee agrees with the recommendations of the ISPD guides of 201614.
Recommendations
- -
We recommend the use of two synergic antibiotics for the treatment of PI due to Pseudomonas spp. and Stenotrophomonas spp. (1B)
- -
We recommend the use of antibiotic combinations for the treatment of polymicrobial PI, in relation to the isolated microorganisms (1B)
- -
In aggressive enterococcal PI treated with vancomycin or ampicillin, we suggest the addition of un intraperitoneal aminoglycoside, provided the antibiogram evidences a synergistic potential (2B)
Peritoneal infection due to CNS is the paradigm of infection resulting from contamination through manual contact during connection or disconnection of the system. These infections are usually not aggressive and typically respond well to any antibiotic with activity against grampositive microorganisms. The CNS infection rates are a good indicator of correct training of the patient.
This Committee agrees with the recommendations of the ISPD guides of 201614 indicating treatment with vancomycin or first or second generation cephalosporins for two weeks. There are some considerations to be taken into account, however:
- 1
Many centers have a high incidence of infections due to methicillin-resistant CNS248. The local epidemiology of each center is decisive for selecting betalactams or vancomycin for the treatment of these infections.
- 2
The main problem with these infections is the risk of relapses, which are closely associated to the capacity of the mentioned bacteria to generate biofilms249. In some cases, it is not easy to know whether the patient presents repeated or recurrent PI (new infections caused by bacteria of the same group) or true relapse. In the first case, retraining of the patients is the most logical measure. In the case of the relapses, different strategies have been evaluated, including longer antibiotic cycles, synergic antibiotic combinations, the use of antibacterials with good biofilm penetration capacity, and washing or sealing with fibrinolytic agents — with inconsistent results, largely attributable to insufficient sample sizes or the use of uncontrolled study designs. The ISPD guides of 201614 do not support the use of fibrinolytic agents, which in their day generated considerable interest. A recent study has suggested combining prolonged treatments with antibiotic associations adding intraluminal alteplase, but here again the sample size was small, and the study lacked a control group250. Some more recent alternatives, such as sealing with antibacterials222, require more extensive experience before conclusions can be drawn. In practice, it is not uncommon for multiple relapses of PI due to CNS to require replacement of the peritoneal catheter. The ISPD guides of 201614 considers catheter replacement to be feasible in a single step under antibiotic coverage. This Committee agrees with such practice, provided the infection is in the remission phase.
- 3
Some species of CNS, including Staphylococcus lugdunensis, exhibit aggressivity similar to that of Staphylococcus aureus, and therefore may require longer treatments251 — though there is not enough information to allow recommendations to be made in this respect.
This Committee agrees with the general considerations of the ISPD guides of 201614, since no new information of interest has been forthcoming. The main points are:
- -
The treatment of PI caused by this microorganism should last at least three weeks, in view of its aggressivity and persistence.
- -
The high frequency of simultaneous catheter-related infection makes it particularly important to screen for this complication. The coexistence of both conditions would make it necessary to consider the convenience of withdrawing the peritoneal catheter.
- -
Infections caused by methicillin-sensitive strains can be treated with a first-generation cephalosporin or vancomycin, while methicillin-resistant strains must be treated with daptomycin or vancomycin.
- -
The benefit of adding rifampicin to conventional therapy is not clear, for despite favorable data from some uncontrolled studies, this antibacterial has undesirable pharmacokinetic effects that can reduce its advantages.
Some additional considerations of this Committee include the following:
- -
Peritoneal infection produced by this microorganism is characterized by a variable clinical presentation, though the disorder may prove very aggressive, with mean mortality rates comparable to those of PI caused by gramnegative microorganisms59. In cases with a severe clinical presentation, some groups prefer to add an aminoglycoside to cephalosporins or vancomycin, at least until the more serious phase has passed. This strategy is not supported by evidence, however.
- -
Although resistance to vancomycin is infrequent in Staphylococcus aureus, there are vancomycin intermediate resistance Staphylococcus aureus (VISA) strains252. These strains show minimum inhibitory concentrations (MICs) in the intermediate range between sensitivity and resistance, and the PI they cause is associated to an increased risk of therapeutic failure. In these cases, the continuous administration of vancomycin via the intraperitoneal route has been recommended252, though the current nomenclature regards these strains as being resistant; the administration of an alternative antibacterial (such as daptomycin) therefore appears to be the best option.
- -
Catheter withdrawal is clearly indicated in PI associated to catheter-related infection in the presence of a wall abscess or life-threatening infection, but should be individualized in uncomplicated cases253.
Streptococcal PI can have different origins, given the widespread distribution of this bacterial genus (skin, oral cavity, upper and lower digestive tract, upper airway). The oral cavity is a probable site, due to its extensive colonization by these microorganisms254, though the frequent presence of streptococci in polymicrobial infections59,255–257 also suggests the possibility of an enteric origin in many cases. The guides of 2016 advocates intraperitoneal ampicillin for two weeks as the optimum treatment. This Committee considers that in view of the usual sensitivity of these germs to routine empirical antibiotic treatment (vancomycin and cephalosporins)59,257, it is equally reasonable to maintain one of the initial antimicrobials (preferably a cephalosporin), in most cases.
EnterococciEnterococcal PI is characterized by a variable clinical course, though the latter is often complicated due to a number of reasons. Firstly, such infections may be of enteric origin, often manifest in a polymicrobial environment, and frequently reflect underlying surgical abdominal processes. Secondly, their antibiotic susceptibility patterns are complex258. Most of the strains are resistant to aminoglycosides at low concentrations, though gentamycin and streptomycin may present synergism if administered in combination with glycopeptides or ampicillin. Furthermore, although resistance is infrequent, enterococci are the grampositive microorganisms most often resistant to glycopeptides. Of the two most common species, infections produced by Enterococcus faecium tend to exhibit susceptibility and a more complicated clinical course258. Lastly, these are bacteria with a strong capacity to neutralize the antibacterial defense mechanisms and protect themselves through biofilms258,259.
The ISPD guides of 201614 suggests vancomycin (plus an aminoglycoside in the case of synergism) for three weeks as the best therapeutic option, with alternative antimicrobial therapies conditioned to susceptibility in the case of strains resistant to vancomycin (ampicillin, linezolid, daptomycin or quinupristin/dalfopristin). This Committee wishes to comment some aspects:
- -
In the case of sensitive strains, ampicillin may be a more appropriate option, with vancomycin or linezolid being held in reserve for resistant strains.
- -
Although the ISPD guides indicate combination therapy only in severe cases, we suggest that synergy with aminoglycosides may be taken advantage of in all cases of enterococcal PI, particularly in patients without residual kidney function.
- -
Not all the aminoglycosides are equally effective against enterococci. Gentamycin and streptomycin exhibit greater efficacy in this context258.
- -
Quinupristin/dalfopristin play a limited practical role in the treatment of these infections, due to scant activity against Enterococcus faecalis and insufficient experience with the use of these drugs244.
Given the established capacity of the genus Corynebacterium to produce recurrent and relapsing PI, this Committee agrees with the suggestion14 of prolonging the treatment of PI with vancomycin during at least three weeks. A recent multicenter observational study260 suggests that two weeks of antibiotherapy may suffice (instead of the three weeks recommended by the ISPD guides), and that a first-generation cephalosporin may be equivalent to the recommended vancomycin. However, in this study, most of the patients initially treated with cefazolin were switched to vancomycin following isolation, with an important risk of bias.
Different grampositive bacilli may be the cause of PI in PD, including microorganisms belonging to the genera Aerococcus, Propionibacterium, Listeria261, Bacillus262 or Microbacterius263, among others. Some of these infections appear to have particular antimicrobial sensitivity or recurrence profiles, but the available experience is limited to isolated clinical cases or very small case series. The treatment regimens therefore must be individualized according to the sensitivities of each microorganism.
EnterobacteriaceaeThe ISPD guides do not individualize peritoneal infections in relation to this family of microorganisms, but contemplate Enterobacteriaceae under the global cases of PI caused by gramnegative microorganisms (only infections due to Pseudomonas spp. are dealt with separately)14. The only recommendation here is to prolong treatment during at least three weeks — a recommendation which this Committee agrees with.
Peritoneal infections due to enterobacteria have a poor prognosis, with rates in terms of therapeutic failure, failures of the PD technique and mortality higher than those of any other grampositive organism except Staphylococcus aureus. This aggressivity is fundamentally explained by three factors:
- -
The intrinsic clinical aggressivity of the infections produced by the different species, including Escherichia coli. This Committee considers that the use of synergic antibiotic combinations could be useful in clinically aggressive cases, but the evidence in favor of this strategy is weak264.
- -
The probability of underlying abdominal processes that generate, maintain and worsen the clinical course265. The risk of these situations is clear in polymicrobial PI. Although clearly less likely in monobacterial presentations, this Committee suggests that such processes may occur and advocate imaging studies in PI due to Enterobacteriaceae presenting a clinically aggressive course.
- -
Progressively more complex antibacterial resistance patterns that often prove difficult to deal with248. The appearance of extended-spectrum betalactamase (ESBL) (particularly Escherichia coli and Klebsiella spp.) and carbapenemase producing strains has been an important cause of concern14. The former can be treated with carbapenems, though the latter often require the prescription of uncommon antibiotics (such as colistin), which nephrologists in PD are usually not familiar with, and the risk of therapeutic failure is high266,267. The increasing introduction of new cephalosporins combined with betalactamase inhibitors may offer a solution to this problem.
This Committee agrees with the main recommendations of the ISPD guides of 201614 on the management of PI due to Pseudomonas spp., which include treatment using two antibiotics with antipseudomonal activity during at least three weeks, and withdrawal of the catheter in the case of concomitant infection of the tunnel or exit site. Thirty-eight percent of the members of the panel consider that monotherapy is also appropriate in non-aggressive PI. Very little further evidence has been forthcoming since the publication of the mentioned guides. A single-center observational study247 has demonstrated no advantage from adding a third antimicrobial (quinolone) to the usual two-drug antibiotic regimen (betalactam and amikacin) for the treatment of infections due to Pseudomonas spp.
The treatment of PI due to microorganisms belonging to the gender Acinetobacter can pose an important challenge. In particular, multiresistance, including resistance to carbapenems, is very frequent, particularly referred to Acinetobacter baumannii — a fact that may increase the risk of therapeutic failure. The background treatment is similar to that of PI due to Pseudomonas spp., but must be individualized in the case of the most multiresistant species. On the other hand, a recent study of the Anzdata registry243 has evidenced reasonable treatment success rates (74%) in these infections, despite a minority use of combined therapies (13%), as well as comparable outcomes after treatment with aminoglycosides, quinolones or ceftazidime. Bacteria of the genus Stenotrophomonas also show important levels of multiresistance, though they tend to be susceptible to cotrimoxazole14. In view of their often complicated clinical course, it seems reasonable to use antibiotic associations in these infections, if allowed by the corresponding sensitivity patterns.
Polymicrobial infectionsThis Committee agrees with the main recommendations of the ISPD guides of 201614 regarding polymicrobial PI:
- -
Infections caused by non-enteric microorganisms should be treated according to the antibiotic susceptibility profile for three weeks.
- -
Infections caused by enteric flora should be treated with broad spectrum antibiotics covering grampositive organisms, enterobacteria and anaerobes, during at least three weeks. The association metronidazole + vancomycin + aminoglycoside or ceftazidime or ceftriaxone seems adequate for this purpose. Individualized surgical assessment is essential.
This Committee wishes to raise some minor comments in this respect:
- -
There is no clear evidence on the required duration of treatment for non-enteric polymicrobial PI. Infections caused by non-aggressive bacteria (CNS or streptococci) could be treated for two weeks. We consider that in general, treatment should last for as long as required by the most aggressive isolated microorganism.
- -
In the case of PI with the presence of enteric flora, this Committee emphasizes the convenience (at least in the first few days) of providing anaerobe coverage as recommended by the ISPD guides, even in the absence of any initially positive isolation, due to the often late growth of these microorganisms. As suggested by the guides, antimicrobials active against enterobacteria and anaerobes (e.g., carbapenems or penicillin - tazobactam) may be used for this purpose in monotherapy.
- -
This Committee underscores that the term “surgical assessment” does not necessarily imply a surgical operation, but rather clinical and imaging assessment. The decision to operate is based on these criteria.
This Committee agrees with the main recommendations of the ISPD guides of 2016 on the treatment of fungal PI14, with some considerations:
- -
Patient management should include withdrawal of the peritoneal catheter. Although immediate withdrawal is the most common practice, some groups prefer to keep it in place during the first days of treatment. The aim is to continue having the intraperitoneal route available for the administration of antifungals and for reducing the inflammatory response — which in theory would reduce damage to the peritoneal membrane. The catheter is subsequently removed, once the infection is in remission, particularly if it proves refractory to treatment. The evidence is scarce and contradictory191,192 and does not allow firm recommendations to be made. In this case the panel of experts was strongly divided in its opinions: 19% of the members did not consider catheter withdrawal to be essential, and opinion was equally divided in favor of immediate withdrawal and of first attempting to induce remission of the infection. In any case, this Committee considers that withdrawal of the catheter should not be delayed in clinically aggressive infections.
- -
A recent retrospective and uncontrolled study has reported acceptable fungal PI healing rates without catheter withdrawal, performing sealing with amphotericin B, in a sample of 11 cases223. The weakness of the evidence does not allow modification of the abovementioned points of view, however.
- -
Antifungal treatment is to be maintained for at least two weeks after withdrawal of the peritoneal catheter. Although amphotericin and flucytosine have been the basis of the treatment of these infections for decades, azole drugs and echinocandins have gradually become predominant in recent years. Amphotericin remains intact in its efficacy against yeasts and filamentous fungi, but is characterized by significant toxicity, poor peritoneal penetration when administered via the intravenous route, and exerts irritant effects when delivered via the intraperitoneal route. The azoles in turn are highly effective, though of the two most commonly used drugs, fluconazole has limited activity against non-albicans Candida species, and the incidence of resistances is significant. Voriconazole has a more consistent effect, and is active against filamentous fungi, but its excipient does not allow prolonged intravenous administration in the presence of renal failure. The echinocandins are very effective and show good penetration into the peritoneal cavity when administered via the intravenous route, though there is little experience with their intraperitoneal administration. There is not enough information to allow recommendations on which antifungal is best, but it is positive for the number of therapeutic alternatives to become expanded268.
The ISPD guides provide no specific recommendations regarding PI caused by mycobacteria14, though a number of considerations are made, with which this Committee agrees — including the logical indication of combined antibiotic treatment during a sufficient period of time. Likewise, emphasis is placed on the need to decide withdrawal of the peritoneal catheter on an individualized basis — though withdrawal is usually required in most cases. No recent information capable of modifying these points of view has been forthcoming.
Negative cultureAlthough they represent 10−30% of all peritoneal infections5, the information on the management of infections with a negative culture result is limited. One of the major difficulties in offering recommendations for these cases of PI is the heterogeneity of the potential underlying causes (Table T4). Accordingly, the suggestions of the ISPD guides of 2016 on PI with a negative culture are based on the opinion of experts14.
Most common causes of negative culture in possible peritoneal infection.
| Cause | Keys to diagnosis |
|---|---|
| Non-infectious peritoneal inflammation (chemical, icodextrin, eosinophilic)(See also Table D5) |
|
| Microorganism with torpid growth or requiring special media (filamentous fungi, mycobacteria) |
|
| Viral (herpesvirus) |
|
| Microorganisms labile in culture |
|
| Active / recent antibiotic treatment |
|
| Insufficiently concentrated sample (obtained after short dwell) |
|
| Sample obtained or stored under inadequate conditions |
|
A recent analysis by the Anzdata group269 of 1760 episodes of PI with a negative culture confirmed the relatively benign prognosis of these infections. Notoriously, the treatment regimens (monotherapy or combined) based on cefazolin showed greater efficacy than those based on vancomycin.
This Committee suggests the following strategy:
- -
If the infection shows evidence of remission by day three, PI due to grampositive organisms should be assumed, and only cephalosporin or vancomycin should be maintained until two weeks of treatment have been completed.
- -
If the infection shows evidence of only slow remission by day three, we should maintain the initial empirical treatment and consider sampling for culture and polymerase chain reaction (PCR) testing for mycobacteria, according to the clinical context.
- -
If the infection proves refractory by day 5, culture should be repeated, with the consideration of alternative antibiotic treatment (daptomycin, carbapenem) and/or withdrawal of the catheter, according to clinical criterion.
Quality of evidence: Moderate
Synthesis of the evidence
Since the publication of the ISPD guides, little evidence capable of modifying the mentioned criteria has been published. The only contributions come from uncontrolled observational studies suggesting the efficacy of vancomycin and cephalosporins for the treatment of streptococcal PI59, the viability of monotherapy in PI due to Acinetobacter spp.243, the possibility of prescribing amphotericin sealing as an alternative to withdrawal of the catheter in fungal PI223, and the possible superiority of cefazolin over vancomycin in the treatment of PI with negative culture269. None of these studies are supported by levels of evidence capable of modifying the previous points of view, however.
From evidence to recommendation
This Committee agrees with the recommendations of the ISPD guides of 201614, with some minor considerations based on opinions and recent uncontrolled data.
Recommendations
- -
For PI due to coagulase-negative staphylococci, we recommend treatment with vancomycin or first or second generation cephalosporins, during a minimum period of two weeks (1B)
- -
For the frequent recurrences or relapses of PI due to coagulase-negative staphylococci, this Committee suggests individualization of the management strategy. If peritoneal catheter replacement is decided, withdrawal and placement should be made simultaneously or with a brief time interval, once remission has been achieved (2B)
- -
We recommend maintaining the treatment of PI due to Staphylococcus aureus for at least three weeks (1C)
- -
We recommend thorough screening of simultaneous catheter-related infection in PI due to Staphylococcus aureus, and the consideration of withdrawal of the peritoneal catheter in PI due to Staphylococcus aureus associated to extensive soft tissue infection or wall abscesses caused by the same microorganism (1B)
- -
We recommend treatment of PI due to methicillin-sensitive strains of Staphylococcus aureus with a first generation cephalosporin or vancomycin, while methicillin-resistant strains should be treated with vancomycin or daptomycin (1C)
- -
We suggest the addition of an aminoglycoside in PI due to Staphylococcus aureus with a clinically aggressive presentation (2C)
- -
We suggest the treatment of streptococcal PI using vancomycin or betalactams (ampicillin or cephalosporin) for at least two weeks from the initially prescribed empirical protocol (2C)
- -
We recommend the treatment of enterococcal PI using ampicillin or vancomycin (plus an aminoglycoside in the case of synergism) for three weeks, with alternative antibiotic treatment according to susceptibility in the case of resistant strains (linezolid, daptomycin) (1C)
- -
We suggest the treatment of PI due to Corynebacterium with vancomycin during at least three weeks (2C).
- -
We recommend the treatment of PI due to Enterobacteriaceae during at least three weeks (1C)
- -
We suggest the use of synergic antibiotic combinations (cephalosporin and aminoglycoside) or carbapenem as treatment for PI due to Enterobacteriaceae with a clinically aggressive presentation (2C)
- -
We recommend the treatment of PI due to Pseudomonas spp. with two antibiotics exhibiting antipseudomonal activity during at least three weeks, with withdrawal of the peritoneal catheter in the case of concomitant infection of the tunnel or exit site (1B)
- -
We recommend the treatment of polymicrobial infections due to non-enteric species for two or three weeks, conditioned by the isolate of greatest aggressivity (1C)
- -
We recommend the treatment of polymicrobial PI with the isolation of enteric organisms using broad spectrum antibiotics covering grampositive species, enterobacteria and anaerobes, during at least three weeks (1C)
- -
We recommend abdominal imaging tests and/or surgical assessment in enteric polymicrobial PI, and in infections caused by Enterobacteriaceae or enterococci with a clinically aggressive, torpid or refractory behavior (1C)
- -
We recommend that PI with negative culture and rapid remission be regarded as grampositive infection, maintaining treatment for two weeks (1C)
- -
In PI with negative culture and a slow response to empirical treatment, we suggest switching to alternative therapy no more than beyond day 5, and to consider withdrawal of the peritoneal catheter (2C)
Quality of evidence: Low
Synthesis of the evidence
Little evidence has appeared capable of modifying the considerations of the ISPD guides of 201614. A recent retrospective, uncontrolled study223 involving 11 patients with PI due to yeasts has reported a reasonable success rate (64%) without withdrawal of the catheter, and performing sealing with amphotericin B.
From evidence to recommendation
This Committee agrees with the recommendations of the ISPD guides of 201614, with some considerations, as described below.
Recommendations
- -
We recommend withdrawal of the peritoneal catheter in PI due to yeasts (1C)
- -
We recommend treatment with antifungals according to antibiotic susceptibility during at least two weeks after withdrawal of the peritoneal catheter, in PI due to yeasts (1C)
- -
We suggest that an azole drug (in sensitive strains) or an echinocandin (alone or in combination until antimicrobial susceptibility has been established) offers a better efficacy-toxicity profile than amphotericin B, alone or combined with flucytosine, in PI due to yeasts (2D).
Quality of evidence: Low
Synthesis of the evidence
Following publication of the ISPD guides of 2016, we have identified only one descriptive study involving 14 cases of PI due to atypical mycobacteria270 that contributes no new information, though it does underscore the already known risk of confusing these germs with diphteroides.
From evidence to recommendation
This Committee agrees with the recommendations of the ISPD guides of 201614, with some considerations, as described below.
Recommendations
- -
We recommend combination therapy for the required duration in PI due to mycobacteria (1C)
- -
We recommend withdrawal of the peritoneal catheter in PI due to mycobacteria, except in clinically mild cases or infections with a rapid response to tuberculostatic drugs (1C)
- -
We suggest systematic screening of infection due to mycobacteria, in PI with negative culture and a torpid or refractory clinical course (2B)
This Committee agrees with the definitions of an atypical course of PI proposed by the ISPD guides of 20057, 2010271 and 201614 (Table D6). In principle, we consider that no changes should be suggested in a standardized international system, though we observe some weaknesses in the definitions (or in their translation into Spanish), which we will comment further below.
Refractory peritoneal infectionIn overall terms, this Committee agrees with the position of the ISPD guides of 201614 regarding the convenience of withdrawing the peritoneal catheter in cases of refractory PI. Although there is no controlled evidence, the general experience is that this measure protects the life of the patient and reduces suffering, while its capacity to preserve the peritoneal membrane with a view to continuing PD is less evident253. We wish to underscore the equivocal nature of the definition of refractory PI according to the ISPD, since it literally contemplates the normalization of peritoneal cellularity by day 5 of specific treatment. We suggest that the term “refractory” be reserved for this context, but consider that cases showing a tendency towards improvement (clearly subsiding pain, negative control cultures, and a clear decrease in cell count with progression towards a monocytic profile) should be regarded as slowly resolving PI. In these circumstances, the decision to withdraw the catheter should be established on an individualized basis, after considering alternative strategies (change/addition of an antimicrobial agent, monitoring of antibiotic levels), assessing the clinical condition of the patient, and considering the risk-benefit ratio of the measure.
Peritoneal infection with relapse or recurrenceThe ISPD guides of 2016 are deliberately ambiguous in establishing recommendations on the management of relapsing, recurrent or repeated PI14, specifying that, in any of these circumstances, withdrawal of the peritoneal catheter should be considered. While in agreement with this recommendation, this Committee wishes to make some observations:
- -
In first relapses of PI, we recommend the checking of possible breaches in the conditions of treatment of the original infection, including potential response-limiting factors (catheter dependency, abdominal disease), poor adherence, non-optimum antibiotherapy or underdosing.
- -
In first relapses of PI characterized by low aggressivity, the panel of experts are unanimous in suggesting that the best option is a new antibiotic cycle, prolonging its duration for at least three weeks and/or administering antimicrobials with good action against biofilms — with preference over direct withdrawal of the catheter.
- -
In first relapses of PI characterized by aggressive behavior, or in second relapses, we recommend considering withdrawal of the peritoneal catheter.
- -
In the case of recurrent or repeated PI, the first step should be to check adherence to the technique on the part of the patient or caregiver.
- -
In the case of recurrent PI due to enteric organisms, it is essential to carefully evaluate the presence of underlying abdominal disease.
The ISPD guides of 2016 do not include a specific section on the approach to catheter-related PI, though multiple observations relevant to this situation are made. It is assumed that stricto sensu, fungal, refractory or relapsing PI should be included in this category, since it is considered that the catheter had been colonized, and that keeping it in place will perpetuate the infection.
In this aspect, this Committee agrees with the recommendations of the ISPD to consider withdrawal of the catheter in fungal, refractory or relapsing PI (see above). The aims are to accelerate recovery, mitigate suffering, reduce mortality and generate a setting in which the patient, if willing, can again attempt treatment with PD. Following withdrawal of the catheter, a period of time (at least two weeks) should be allowed until insertion of a new catheter. However, when the catheter is removed during a period of remission of PI, simultaneous withdrawal and reinsertion can be decided. This strategy in general affords good results in PI due to CNS14, and has been attempted (with less consistent success) in PI due to more aggressive species such as Staphylococcus aureus272 or Pseudomonas spp.273. This Committee supports this strategy in PI due to CNS, but not in the other mentioned cases.
In the case of PI related to infection of the exit site or tunnel of the peritoneal catheter, the ISPD guides of 2016 are not particularly explicit. It is necessary to refer to the ISPD guides of 201725 on catheter-related infection. In this scenario, the suggestion is to consider withdrawal of the catheter, allowing a minimum time of two weeks before inserting a new catheter. This strategy is particularly convenient in the case of infection produced by aggressive and/or persistent organisms such as Staphylococcus aureus, non-fermenting gramnegative bacilli or enterobacteria (Serratia spp., Proteus spp. and others)272. This Committee agrees with this approach, underscoring that the conservative management of these infections is possible, provided there are no complicating elements (refractory or slowly resolving PI, wall abscesses, or refractory or recurrent catheter-related infection).
There is no recent evidence to modify the considerations of the ISPD guides of 2016 14. The observational studies published by Szeto274 and Burke et al.275 remain as the only valid references for guiding the management of these cases. Following these publications, we have only identified two small single-center observational and descriptive studies that offer no further information of relevance276,277.
Quality of evidence: Low
Synthesis of the evidence
There is no recent evidence to modify the considerations of the ISPD guides of 201614.
From evidence to recommendation
This Committee agrees with the recommendations of the ISPD guides of 201614, with some considerations that are described below.
Recommendations
- -
We recommend withdrawal of the peritoneal catheter in cases of totally refractory PI (1C)
- -
We suggest that, in slowly resolving PI (decreasing activity on day 5 of treatment), the optimization of antimicrobial treatment should be decided before considering withdrawal of the catheter (2C)
- -
We recommend withdrawal of the peritoneal catheter in aggressive relapses of PI, or in second relapses of any PI (1C)
- -
We suggest prolonged and optimized antimicrobial treatment for the initial management of non-aggressive first relapses of PI (2C)
- -
We recommend investigation of the possible causes of recurrent PI, including poor adherence to the technique or occult abdominal conditions (1C)
- -
We suggest considering withdrawal of the peritoneal catheter within the strategy for dealing with recurrent PI (2C)
Quality of evidence: Low
Synthesis of the evidence
The ISPD guides of 201614 make little direct mention of this issue, which nevertheless is discussed in the guides of 2017 on catheter-related infection25. We have identified no more recent articles affording further evidence of value in this respect.
From evidence to recommendation
This Committee aggress with the recommendations of the ISPD guides of 201614 and 201725, with minor comments.
Recommendations
- -
We recommend withdrawal of the peritoneal catheter in PI associated to catheter-related infection produced by the same microorganism, in the following cases: refractory PI, slowly resolving PI, relapsing PI, PI associated to wall abscess, and PI associated to catheter-related infection characterized by slow resolution or recurrence (1C)
- -
We suggest considering withdrawal of the catheter in the rest of cases of simultaneous infection of the catheter produced by the same microorganism (2C)
- -
We suggest a period of at least two weeks between withdrawal of the catheter and the reinsertion of a new catheter, in PI associated to catheter-related infection produced by the same microorganism (2C)
Quality of evidence: Low
Synthesis of the evidence
There is no recent evidence to modify the considerations of the ISPD guides of 201614.
From evidence to recommendation
This Committee agrees with the recommendations of the ISPD guides of 2016 14.
Recommendations
- -
We recommend a period of at least two weeks between withdrawal of the catheter due to PI and the insertion of a new catheter (1C)
- -
We recommend that insertion of a new catheter should not be attempted if there are persistent signs of peritoneal irritation (1C)
- -
We suggest that simultaneous withdrawal and insertion of a peritoneal catheter in PI is feasible, provided the infection is in complete remission and is caused by non-aggressive microorganisms (2B)
The intraperitoneal dosing specifications are provided in Table T514,237,240,244,278,279. It is important to point out the few published controlled studies facilitating reliable dosing definitions. Most of the recommendations come from uncontrolled observational studies, and many are based on extrapolations of the corresponding intravenous doses. Furthermore, in general, no consideration is made of important parameters such as body size, patient gender, residual kidney function or the PD regimen used. Fortunately, much empirical experience has been gained in the use of the most common antibiotics (vancomycin, cephalosporins, carbapenems and aminoglycosides), allowing nephrologists in PD to prescribe doses of these antimicrobials with relative confidence.
Tentative dosing of intraperitoneal antibiotherapy.
| Antibiotic | Daily intermittent dose | Continuous dose | Observations |
|---|---|---|---|
| Amikacin | 2 mg/kgLD | 12 mg/lLD | Continuous not recommendedMonitoring of levels advisedRKF |
| Gentamycin | 0.6 mg/kgLD | 4 mg/lLD | Continuous not recommendedMonitoring of levels advisedRKF |
| Tobramycin | 0.6 mg/kgLD | 10 mg/lLD | Continuous not recommendedMonitoring of levels advisedRKF |
| Cefazolin | 15−20 mg/kg | 125 mg/lLD | |
| Cefepime | 1000 mg | 100−125 mg/lLD | As intermittent can be administered every 48 hPotential stability problems in dialysate |
| Cefotaxime | 500−1000 mg | 125−250 mg/lLD | Insufficient evidence in continuous dosingRKF |
| Ceftazidime | 1000−1500 mg | 125 mg/lLD | As intermittent can be administered every 48 hPotential stability problems in dialysateRKF |
| Ceftriaxone | 1000 mg | No data | |
| AmpicillinAmpicillin-Sulbactam | 500−1000 mg1000 mg x 2 | 125 mg/l100 mg/lLD | Potential stability problems in dialysateRKF |
| Piperacillin-Tazobactam | 2000 mg x 2 | 500 mg/lLD | RKF |
| Aztreonam | 500−1000 mg x 2 | 125−250 mg/lLD | RKF |
| Ciprofloxacin | 500 mg | 50 mg/l | Intermittent not recommended due to lack of information |
| Ofloxacin | 100 mg | 25 mg/lLD | Intermittent not recommended due to lack of information |
| Daptomycin | 250−350 mgLD | 20 mg/lLD | As intermittent can be administered every 48 hPotential stability problems in dialysateIcodextrin interferes with determination of levels |
| Linezolid | No data | No data | Stable in PD solutions, but not advised due to lack of clinical experience |
| Quinupristin/Dalfopristin | 25 mg/l x 2LD | No data | |
| Imipenem-Cilastatin | 500 mg x 2 | 50−125 mg/lLD | Potential stability problems in dialysateRKF |
| Meropenem | 1000 mg | 125 mg/l | Little experience with continuous dosingRKF |
| Vancomycin | 15−30 mg/kg | 25−150 mg/lLD | Monitoring of levels advisedRKF |
| Teicoplanin | 15−30 mg/kg | 10−50 mg/lLD | Monitoring of levels advisedRKF |
| Cotrimoxazole | No data | 40/200 mg/lLD | Little experience |
| Fluconazole | 200 mg | 25−50 mg/l | RKF |
| Voriconazole | 2.5 mg/kg | No data | Risk of accumulation of excipient (SBECD) after prolonged parenteral administration |
LD: administer initial loading dose; RKF: pay special attention to residual kidney function for dosing.
It is also important to mention the great variability in the results of the studies that analyze the stability of antibiotics in PD solutions. In general, the most widely used drugs maintain their activity beyond 24 h, and some do so for many days278. However, a recent study279 has questioned the stability of amoxicillin, cefepime, imipenem and ceftazidime, and does not recommend storage periods of more than 24 h for vancomycin, cefazolin, tobramycin and cotrimoxazole. The centers that provide patients with PI with dialysate bags containing antibiotics should take this into account.
Proposals for study and improvementThis guide once again evidences the scarcity and low quality of the scientific evidence available in the field of Peritoneal Dialysis. Clinical practices that have become fully consolidated over time in fact lack robust confirmation of their benefits or adverse effects. A number of preventive measures or antibiotic prescription policies cause bewilderment and sometimes manifest disagreement among microbiologists and specialists in infectious diseases. In the absence and in wait of better evidence, we have tried to reach a balance between the recommendations of general orthodox practice in infectious diseases and the current recommendations of the ISPD guides, proposed by successive expert committees, that have been warranted by practice and not disqualified by alternative strategies. The design of this guide, adhering to the GRADE system, seeks to periodically integrate new information, with a view to adding to or improving the quality of our knowledge.
In general, the panel of experts considers that more randomized clinical trials are needed to resolve the many controversial issues related to the prevention and treatment of PI. Some of the aspects that have specifically focused our attention have been the following:
- -
The convenience of adopting a common registry at Spanish national level (with the participation of physicians and nursing staff in PD), allowing us to advance more in depth in our approach to these infections, in a way similar to what has been achieved in the Anzdata setting.
- -
The systematic application of combined prevention strategies (Continuous Quality Improvement [CQI]).
- -
The clinical usefulness of emerging techniques for the early/rapid diagnosis and prediction of the risk of PI relapse
- -
Improvements in connectology, especially for patients with disabilities.
- -
Improvement of the scarce and controversial data on the optimum short- and long-term care of the exit site of the peritoneal catheter, including the convenience of using topical antibacterials and their form of application.
- -
Definition of treatment protocols for carriers of Staphylococcus aureus.
- -
The true usefulness of PI prevention protocols in patients subjected to invasive procedures
- -
The role of changes in the intestinal and general microbiota of patients in the epidemiology and prevention of PI in PD.
- -
The role of antibacterial sealing for the prevention and treatment of complicated PI.
- -
The epidemiology and treatment of PI due to multiresistant microorganisms.
- -
Indications and clinical usefulness of imaging tests in PI with an atypical course.
- -
Definition of criteria on the timing of withdrawal of the peritoneal catheter in PI due to yeasts.
- -
More complete information on the pharmacokinetics and toxicity of new antibacterials.
- -
The need for common PI registries in the new indications of PD (heart failure, liver disease with ascites).
- -
Definition of regimens for the prevention and treatment of PI in the new indications of the PD (heart failure, liver disease with ascites).
- 1
Peritoneal infection should be suspected in the presence of abdominal pain and/or a turbid (cloudy) peritoneal effluent. The diagnosis is to be confirmed by cytological and microbiological study of the effluent
- 2
The identification of an abnormally elevated leukocyte count (>100/mm3) with a dominant presence of polymorphonuclear cells (>50%) in the effluent is strongly suggestive of the presence of PI
- 3
The diagnosis of PI should be individualized in relation to the clinical circumstances, based on the criteria described in the above sections
- 4
Careful initial assessment, with correct collection and management of samples, is fundamental for ensuring PI treatment success
- 5
Systematic microbiological analysis of the effluent is required based on gram staining, within the initial diagnostic process of PI
- 6
Each center should compile evolutive and up to date information on the PI rates (overall and according to causal pathogens), using standardized estimators, and at least once a year
- 7
Ideally, the monitored parameters should include the overall PI rate, the PI rates according to specific microorganisms, the percentage of patients/year that remain free of PI, and the antimicrobial resistances of the causal pathogens
- 8
The best way to report the PI rates is as the number of episodes per patient and year
- 9
Similar diagnostic criteria for PI are to be applied in patients subjected to manual or automated PD
- 10
It is crucial to systematically monitor PI through clinical, cytological and microbiological controls, until a clearly favorable course is confirmed
- -
We recommend each PD Unit to develop a Continuous Quality Improvement program in order to reduce the incidence of PI (1C) (Question P1)
- -
We suggest periodic meetings of the multidisciplinary teams in charge of the continuous quality improvement program in order to analyze the outcomes (2C) (Question P1)
- -
We recommend the patient training and monitoring principles proposed by the documents of the ISPD in order to reduce the PI rates (1C)(Question P2)
- -
We recommend the nursing staff in charge of patient training and monitoring to be specialized (1C) (Question P2)
- -
We suggest that visits to the home of the patient, if possible, beginning before the start of treatment with PD, may help reduce the risk of PI (2C) (Question P2)
- -
We suggest that retraining under indication could help reduce the incidence of PI in situations of risk (2C) (Question P2)
- -
Based on the available evidence, this Committee does not endorse programmed retraining for reducing the incidence of PI (2C) (Question P2)
- 1
We recommend the administration of prophylaxis with systemic antibiotics immediately before insertion of the peritoneal catheter (1A)(Question P3)
- 2
We suggest that each PD program should choose its own antibiotic prophylaxis regimen, considering the local incidences and antibiotic sensitivities (2D) (Question P3)
- 3
We suggest vancomycin as a preferential option, though first or second generation cephalosporins may afford equivalent results (2B) (Question P3)
- 4
We recommend that the risk of PI should not be a consideration when deciding which insertion technique or peritoneal catheter design to be used in a patient on PD (1A)(Question P3)
- 5
We recommend regular monitoring and care of the exit site of the peritoneal catheter, if possible on a daily basis, as an indirect way to prevent PI (1C)(Question P4)
- 6
We recommend early treatment of CRI, in order to prevent progression to PI (1C) (Question P4)
- 7
We suggest the use of immobilization systems to avoid repeated traction upon the peritoneal catheter, at least during the post-implantation healing phase (2C) (Question P4)
- 8
We suggest that each center should decide the type of soap or topical antiseptic for care of the catheter exit site, in accordance with the conditions of each patient (2C) (Question QP4)
- 9
We suggest that each center should decide the type of care or dressing of the catheter orifice (open or closed), in accordance with the conditions of each patient, as there is no evidence generally supporting any specific type for preventing CRI and PI (2C) (Question P4)
- 10
We recommend the administration of topical mupirocin or gentamycin during care of the exit site of the peritoneal catheter, as a way to reduce the risk of CRI and PI (1B)(Question P5)
- 11
We suggest the convenience of periodic monitoring of the possible appearance of bacterial resistances to mupirocin or gentamycin among the bacterial flora (particularly Staphylococcus aureus) colonizing the nasal passages and the peri-catheter area (2B) (Question P5)
- 12
This Committee provides no recommendation on the convenience of daily versus intermittent administration of the mentioned antimicrobials (ungraded) (Question Q5)
- 13
This Committee considers that the evidence does not warrant the treatment of nasal carriers of Staphylococcus aureus to reduce the direct risk of PI, though treatment may prove to be of benefit through reduction of the risk of CRI (2D) (Question P5)
- 1
We recommend that PD always should be performed in a clean environment, preferably with nearby access to running water (1C)(Question P6)
- 2
We recommend correct hygiene before performing PD, with washing of the hands using common or antibacterial soap, followed by rubbing of the hands with a water-alcohol solution (1C)(Question P6)
- 3
We recommend avoiding physical contact between domestic pets and the materials used for PD. In addition, patients should maximize asepsis and disinfection if coming into contact with pets (1C) (Question P6)
- 4
This Committee suggests the convenience of wearing a facemask for exchange, particularly in patients with poor dental and periodontal health (2C) (Question P6)
- 1
We recommend the use of systems with disconnection and purging before filling as the option of choice for connection in PD (1A)(Question P7)
- 2
We suggest that double bag systems are preferable to the double-connection Y-set systems (2B)(Question P7) (little used in Spain)
- 3
We recommend that the prevention of PI should not be included among the indications for treatment with APD (1B)(Question P8)
- 4
We suggest that the use biocompatible solutions has no confirmed effect upon the risk of PI (2A)(Question P9)
- 1
We recommend that patients should be specifically instructed during training in PD on how to act in the event of accidental disruption of asepsis (1C)(Question P10)
- 2
We suggest that “wet” contamination or contamination of an uncertain nature should be dealt with by replacing the connection line and administering antibiotic coverage via the oral or parenteral (intravenous or intraperitoneal) route, and covering both grampositive and gramnegative organisms, with a minimum duration of two days (2C)(Question P10)
- 3
We recommend that “dry” contamination at least should dealt with by replacing the connection line (2D)(Question P10)
- 4
We recommend that patients on PD scheduled for invasive biliary, lower gastrointestinal or gynecological procedures should receive appropriate antibiotic prophylaxis (1C)(Question P11)
- 5
We suggest that the provided antibiotic prophylaxis should be active against common enterobacteria and intestinal anaerobes (2C) (Question P11)
- 6
We suggest that antibiotic prophylaxis is not necessary in endoscopic upper gastrointestinal tract explorations (2C) (Question P11)
- 7
We suggest that the available information does not allow us to establish the convenience of voiding the peritoneal cavity for the abovementioned procedures (2C) (Question P11)
- 8
We suggest that routine antibiotic prophylaxis be considered for dental procedures, especially those with a greater bacteremic potential (ungraded) (Question P11)
- 9
We recommend that patients on PD administered oral or parenteral antibiotic treatment should receive oral antifungal prophylaxis during the treatment period (1B)(Question P12)
- 10
We suggest that azoles may be the most appropriate choice for this purpose, though nystatin may be an adequate alternative (2C)(Question P12)
- 11
We suggest the convenience of maintaining adequate patient plasma vitamin D levels (with the prescription of supplements if needed), in order to reduce the general risk of infection in patients subjected to PD (2D)(Question P13)
- 1
We recommend that antibiotherapy be started as soon as possible when PI is suspected (1C)(Question T1)
- 2
We recommend adequate coverage of grampositive and gramnegative microorganisms as part of the initial PI treatment protocol, in all cases (1C) (Question T1)
- 3
We recommend adjusting the empirical treatment protocol for PI to the local epidemiology (1C) (Question T1)
- 4
In the case of centers without sufficient experience of their own, we recommend vancomycin or a first-generation cephalosporin to cover grampositive microorganisms, and a third-generation cephalosporin or an aminoglycoside to cover gramnegative microorganisms (1B) (Question T1)
- 5
We suggest limiting the use of aminoglycosides in patients with PI and significant residual kidney function (2C) (Question T1)
- 6
We suggest the start of treatment for PI using antibiotic doses at the upper limit of the range recommended by the Summary of Product Characteristics, taking into account the residual kidney function of the patient (2C)
- 7
We suggest the convenience of having alternative antibiotic protocols for patients with a history of allergies or intolerances to antibiotics, particularly betalactam drugs (2C) (Question T1)
- 1
We recommend peritoneal lavage in the case of very turbid peritoneal drainage and/or intense abdominal pain, with the aim of providing pain relief and improving the mechanics of PD (1C)(Question T2)
- 2
We suggest the addition of heparin to the dialysate in patients with a turbid effluent, in order to maintain good mechanics of PD (2C)(Question T2)
- 1
We recommend the intraperitoneal route as the preferred option for the treatment of PI on an ambulatory basis, provided the level of training of the patient is adequate (1B)(Question T3)
- 2
In hospitalized patients, we likewise suggest the intraperitoneal route as the preferred option, though the intravenous route is also considered adequate – the decision being conditioned to the pharmacokinetic properties of the antibiotic employed and the clinical context (2B)(Question T3)
- 3
We recommend that the treatment of PI should not be based on monotherapy via the oral route (1C)(Question T3)
- 4
We recommend that intraperitoneal aminoglycosides should be administered on an intermittent basis for the treatment of PI in PD (1B) (Question T4)
- 5
We recommend that the intraperitoneal administration of vancomycin be prescribed on either a continuous or an intermittent basis (1B) (Question T4)
- 6
We suggest that whenever possible, the plasma vancomycin and aminoglycoside levels should be monitored, especially in patients with residual kidney function (2B) (Question T4)
- 7
We suggest that the intraperitoneal administration of betalactams and carbapenems be prescribed on either a continuous or an intermittent basis – the decision being conditioned by the normal dosing interval of each antibiotic (2C) (Question T4)
- 8
In patients on APD with PI treated using intraperitoneal antibiotics, we recommend the administration of vancomycin and betalactams on a continuous basis in order to avoid subtherapeutic drug levels in the dialysate during the short nocturnal dwells (1B) (Question T5)
- 9
When choosing an intermittent intravenous, intraperitoneal or oral antibiotic administration regimen in patients on APD, we recommend the application of general criteria similar to those used in CAPD (1B) (Question T5)
- 10
In patients on APD with PI, we recommend that in the case of intermittent intraperitoneal administration, the latter should be carried out in the course of a long exchange, with a dwell of at least 6 h (1B) (Question T5)
- 11
In patients with a nocturnal intermittent PD regimen (dry day) to be treated via the intraperitoneal route, we suggest the temporary introduction of a diurnal exchange to facilitate administration (intermittent or continuous) of the antimicrobial treatment (2D) (Question T5)
- 12
We suggest that the practice of transferring patients on APD to CAPD during the treatment of PI via the intraperitoneal route is not justified by therapeutic reasons, though it may be justified on a practical basis (2D) (Question T5)
- 1
We recommend a minimum duration of 14 days for the antibiotic treatment of PI in PD (1B) (Question T6)
- 2
We suggest that it may be advisable to prolong treatment at least three weeks in PI due to Staphylococcus aureus, Corynebacterium spp., Enterococcus spp., gramnegative bacteria and mixed flora, with the participation of enteric organisms (2B) (Question T6)
- 3
We suggest prolongation of the duration of treatment for at least one additional week when clinical criteria indicate an increased risk of a complicated course of PI (2C) (Question T6)
- 4
We recommend the use of two synergic antibiotics for the treatment of PI due to Pseudomonas spp. and Stenotrophomonas spp. (1B) (Question T7)
- 5
We recommend the use of antibiotic combinations for the treatment of polymicrobial PI, in relation to the isolated microorganisms (1B) (Question T7)
- 6
In aggressive enterococcal PI treated with vancomycin or ampicillin, we suggest the addition of un intraperitoneal aminoglycoside, provided the antibiogram evidences a synergistic potential (2B) (Question T7)
- 1
For PI due to coagulase-negative staphylococci, we recommend treatment with vancomycin or first or second generation cephalosporins during a minimum period of two weeks (1B) (Question T8)
- 2
For the frequent recurrences or relapses of PI due to coagulase-negative staphylococci, this Committee suggests individualization of the management strategy. If peritoneal catheter replacement is decided, withdrawal and placement should be made simultaneously or with a brief time interval, once remission has been achieved (2B) (Question T8)
- 3
We recommend maintaining the treatment of PI due to Staphylococcus aureus for at least three weeks (1C) (Question T8)
- 4
We recommend thorough screening of simultaneous catheter-related infection in PI due to Staphylococcus aureus, and the consideration of withdrawal of the peritoneal catheter in PI due to Staphylococcus aureus associated to extensive soft tissue infection or wall abscesses caused by the same microorganism (1B) (Question T8)
- 5
We recommend treatment of PI due to methicillin-sensitive strains of Staphylococcus aureus with a first generation cephalosporin or vancomycin, while methicillin-resistant strains should be treated with vancomycin or daptomycin (1C) (Question T8)
- 6
We suggest the addition of an aminoglycoside in PI due to Staphylococcus aureus with a clinically aggressive presentation (2C) (Question T8)
- 7
We suggest the treatment of streptococcal PI using vancomycin or betalactams (ampicillin or cephalosporin) for at least two weeks from the initially prescribed empirical protocol (2C) (Question T8)
- 8
We recommend the treatment of enterococcal PI using ampicillin or vancomycin (plus an aminoglycoside in the case of synergism) for three weeks, with alternative antibiotic treatment according to susceptibility in the case of resistant strains (linezolid, daptomycin) (1C)
- 9
We suggest the treatment of PI due to Corynebacterium with vancomycin during at least three weeks (2C) (Question T8)
- 10
We recommend the treatment of PI due to Enterobacteriaceae during at least three weeks (1C) (Question T8)
- 11
We suggest the use of synergic antibiotic combinations (cephalosporin and aminoglycoside) or carbapenem as treatment for PI due to Enterobacteriaceae with a clinically aggressive presentation (2C) (Question T8)
- 12
We recommend the treatment of PI due to Pseudomonas spp. with two antibiotics exhibiting antipseudomonal activity during at least three weeks, with withdrawal of the peritoneal catheter in the case of concomitant infection of the tunnel or exit site (1B) (Question T8)
- 13
We recommend the treatment of polymicrobial infections due to non-enteric species for two or three weeks, conditioned by the isolate of greatest aggressivity (1C)(Question T8)
- 14
We recommend the treatment of polymicrobial PI with the isolation of enteric organisms using broad spectrum antibiotics covering grampositive species, enterobacteria and anaerobes, during at least three weeks (1C) (Question T8)
- 15
We recommend abdominal imaging tests and/or surgical assessment in enteric polymicrobial PI, and in infections caused by Enterobacteriaceae or enterococci with a clinically aggressive, torpid or refractory behavior (1C) (Question T8)
- 16
We recommend that PI with negative culture and rapid remission be regarded as grampositive infection, maintaining treatment for two weeks (1C) (Question T8)
- 17
In PI with negative culture and a slow response to empirical treatment, we suggest switching to alternative therapy no more than beyond day 5, and to consider withdrawal of the peritoneal catheter (2C) (Question T8)
- 18
We recommend withdrawal of the peritoneal catheter in PI due to yeasts (1C)(Question T9)
- 19
We recommend treatment with antifungals according to antibiotic susceptibility during at least two weeks after withdrawal of the peritoneal catheter, in PI due to yeasts (1C)(Question T9)
- 20
We suggest that an azole drug (in sensitive strains) or an echinocandin (alone or in combination until antimicrobial susceptibility has been established) offers a better efficacy-toxicity profile than amphotericin B, alone or combined with flucytosine, in PI due to yeasts (2D)(Question T9)
- 21
We recommend combination therapy for the required duration in PI due to mycobacteria (1C)(Question T10)
- 22
We recommend withdrawal of the peritoneal catheter in PI due to mycobacteria, except in clinically mild cases or infections with a rapid response to tuberculostatic drugs (1C)(Question T10)
- 23
We suggest systematic screening of infection due to mycobacteria, in PI with negative culture and a torpid or refractory clinical course (2B)(Question T10)
- 1
We recommend withdrawal of the peritoneal catheter in cases of totally refractory PI (1C) (Question T11)
- 2
We suggest that in slowly resolving PI (decreasing activity on day 5 of treatment), the optimization of antimicrobial treatment should be decided before considering withdrawal of the catheter (2C) (Question T11)
- 3
We recommend withdrawal of the peritoneal catheter in aggressive relapses of PI, or in second relapses of any PI (1C) (Question T11)
- 4
We suggest prolonged and optimized antimicrobial treatment for the initial management of non-aggressive first relapses of PI (2C) (Question T11)
- 5
We recommend investigation of the possible causes of recurrent PI, including poor adherence to the technique or occult abdominal conditions (1C) (Question T11)
- 6
We suggest considering withdrawal of the peritoneal catheter within the strategy for dealing with recurrent PI (2C) (Question T11)
- 7
We recommend withdrawal of the peritoneal catheter in PI associated to catheter-related infection produced by the same microorganism, in the following cases: refractory PI, slowly resolving PI, relapsing PI, PI associated to wall abscess, and PI associated to catheter-related infection characterized by slow resolution or recurrence (1C)(Question T12)
- 8
We suggest considering withdrawal of the catheter in the rest of cases of simultaneous infection of the catheter produced by the same microorganism (2C)(Question T12)
- 9
We suggest a period of at least two weeks between withdrawal of the catheter and the reinsertion of a new catheter, in PI associated to catheter-related infection produced by the same microorganism (2C)(Question T12)
- 10
We recommend a period of at least two weeks between withdrawal of the catheter due to PI and the insertion of a new catheter (1C)(Question T13)
- 11
We recommend that insertion of a new catheter should not be attempted if there are persistent signs of peritoneal irritation (1C)(Question T13)
- 12
We suggest that simultaneous withdrawal and insertion of a peritoneal catheter in PI is feasible, provided the infection is in complete remission and is caused by non-aggressive microorganisms (2B)(Question T13).
This guideline received funding from a competitive grant of the Spanish Society of Nephrology (Ayuda para la realización de guías de práctica clínica, documentos de consenso y tomas de posición 2018) (GPC-DC/TP S.E.N.). In addition, the Guideline recieved indirect financial support, partial and unconditioned , from Baxter Renal Care, provided through SENEFRO Foundation.
Conflict of interestNone.
Miguel Pérez-Fontán (miguel.perez.fontan@sergas.es).
Degree in Medicine, Universidad de Santiago de Compostela (1980). Specialist in Nephrology after resident training in Hospital La Paz (Madrid) (1986). Nephrologist at Hospital Universitario de A Coruña, since 1986. Doctor of Medicine, Universidad de A Coruña (1997). Assistant Professor in Nephrology. Experience in peritoneal dialysis since 1983, with many publications.
The author declares that he/she has no conflicts of interest in relation to the present Guide.
Mercedes Moreiras-Plaza (Maria.Mercedes.Moreiras.Plaza@sergas.es).
Degree in Medicine (Universidad de Santiago)(1987). Resident in training in Nephrology (Hospital Universitario de Vigo (1989-1992). Nephrologist (Hospital Universitario de Vigo)(1996). Peritoneal Dialysis Unit, with many communications at congresses and publications in the field.
The author declares that he/she has no conflicts of interest in relation to the present Guide.
Mario Prieto-Velasco (mprietov@gmail.com).
Degree in Medicine, Universidad de Sevilla (1987). Doctor of Medicine, Universidad de Cantabria (1997). Resident in training, Hospital Marqués de Valdecilla, Santander (1988-92). Head of the Department of Nephrology, Hospital de León (since 2013).
The author declares that he/she has no conflicts of interest in relation to the present Guide.
Carlos Quereda Rodríguez-Navarro (carlosqueredam@gmail.com).
Degree in Medicine, Universidad de Granada. Resident in training, Hospital Puerta de Hierro (Nephrology and Internal Medicine). Head of the Department of Nephrology, Hospital Ramón y Cajal de Madrid (2009-2015). Professional activity predominantly in Clinical Nephrology. Member of the GLOSEN s Group since 2005. Workshop on Teaching Evidence Based Health Care. UCL London Medical School 1998. Medical Subdirector of Teaching and Research, Head of Studies, Patron of the Fundación para la Investigación Biomédica, Member of the Steering Committee of the “Ramón y Cajal” Institute of Health Research and Technical Consultant of its Steering Committee (IRYCIS). VII National Award for Healthcare Innovation and Management (Special Award for Research Management) of HRC, Madrid 2000. Associate Professor of Medicine, Universidad de Alcalá. Publications: 190 international and 224 in Spanish. Honorary Member of the S.E.N. 2013; Vice-Chairman of the National Commission for the Specialty of Nephrology 2007 to 2014; Member of the Research Commission, S.E.N. Director of the journal Nefrología 2007 to 2013. Coordinator of the Nephrology Based on Evidence Group of the S.E.N. since 2005. Patron of the SENEFRO Foundation since 2015. Editor of Nefrología y Nefrología al Día, to date. Since his retirement (2015), he collaborates in the development of Guides and Consensus Documents at the S.E.N.
The author declares that he/she has no conflicts of interest in relation to the present Guide.
María Auxiliadora Bajo-Rubio (mabajo.hulp@salud.madrid.org).
Degree in Medicine, Universidad de Salamanca (1987). Doctor of Medicine (UAM 1996). Specialist in Nephrology (Resident in training), Hospital Universitario La Paz (1992). Section Chief of Nephrology, Hospital Universitario La Paz (2013). Acting Head of the Department de Nephrology, Hospital Universitario La Paz (2021).
The author declares that he/she has no conflicts of interest in relation to the present Guide.
Mercè Borràs-Sans (mmborras.hj23.ics@gencat.cat).
Degree in Medicine and Surgery, Universidad Autónoma de Barcelona (1987). Doctor of Medicine (UAB, 1994). Specialist in Nephrology (Resident in training), Hospital Universitari Germans Trias i Pujol (1992). Section Chief of Nephrology, Hospital Universitari Arnau de Vilanova de Lleida (2004). Acting Head of the Department of Nephrology, Hospital Universitari Joan XXIII de Tarragona (2019).
The author declares that he/she has no conflicts of interest in relation to the present Guide.
Verónica of the Espada-Piña (veronica.espadap@gmail.com)
Degree in Medicine, Universidad de Cádiz (2013). Doctor of Medicine, Universidad de Cádiz (2021). Resident in training in Nephrology, Hospital Universitario de Puerto Real (2014-2018). Specialist in Nephrology, Area of Peritoneal Dialysis, since 2019.
The author declares that he/she has no conflicts of interest in relation to the present Guide.
Javier Pérez-Contreras (perez_fra@gva.es).
Degree in Medicine, Universidad de Valencia (1982). Doctor of Medicine, Universidad de Alicante (1997). Resident in training in Nephrology, Hospital General de Asturias (1985-88). Head of the Department de Nephrology, Hospital General Universitario de Alicante (2015).
The author declares that he/she has no conflicts of interest in relation to the present Guide.
Gloria del Peso-Gilsanz (gloria.delpeso@salud.madrid.org).
Degree in Medicine, Universidad Complutense de Madrid (1991). Doctor of Medicine, Universidad Autónoma de Madrid (2014). Resident in training in Nephrology, Hospital Universitario La Paz (Madrid)(1992-95). Specialist in Nephrology, Hospital Universitario La Paz de Madrid (since 1998).
The author declares that he/she has no conflicts of interest in relation to the present Guide.
Esther Ponz-Clemente (eponz@tauli.cat)
Degree in Medicine and Surgery (Universidad de Barcelona)(1985). Doctor of Medicine, Universidad de Barcelona (1992). Specialist in Nephrology (Resident in training), Hospital Clínic de Barcelona (1986-1989). Staff physician in Nephrology, Parc Taulí Sabadell, Hospital Universitari, since May 1991. Senior Medical Consultant (Level D) Parc Taulí Sabadell, Hospital Universitari, since 2016.
The author declares that he/she has no conflicts of interest in relation to the present Guide.
César Remón-Rodríguez (cesarkai@telefonica.net)
Degree in Medicine (Universidad de Sevilla)(1975). Doctor of Medicine, Universidad de Cádiz (2009). Chairman of the Sociedad Andaluza de Nefrología (2012-15). Associate Professor of Health Sciences, Universidad de Cádiz (1987-). Head of the Department of Nephrology (Hospitales Universitarios de Cádiz)(2010-). Extensive experience and numerous publications in Nephrology en general, and in Peritoneal Dialysis in particular.
The author declares that he/she has no conflicts of interest in relation to the present Guide.
Emilio Sánchez-Alvarez (jesastur@hotmail.com).
Degree in Medicine (Universidad de Oviedo)(1991). Doctor of Medicine, Universidad de La Laguna (2008). Resident in training, Hospital Universitario Central de Asturias, 1992-1996. Head of the Department de Nephrology, Hospital Universitario de Cabueñes (Gijón, Principado de Asturias).
The author declares that he/she has no conflicts of interest in relation to the present Guide.
Nicanor Vega-Rodríguez (nvegdia@gobiernodecanarias.org).
Degree in Medicine, Universidad de La Laguna (1981). Doctor of Medicine, Universidad de Sevilla (1992). Resident in training in Nephrology, Hospital Universitario Virgen Macarena. Sevilla (1982-1985). Section Chief of Nephrology, Hospital Universitario de Gran Canaria Dr. Negrín, 2015.
The author declares that he/she has no conflicts of interest in relation to the present Guide.
Manel Vera-Rodríguez (MVERA@clinic.cat).
Degree in Medicine, Universitat de Lleida (1995). Doctor of Medicine, Universitat de Barcelona (2020). Resident in training, Hospital Clínic de Barcelona (1997-2001). Supervisor, Peritoneal Dialysis Unit, Hospital Clínic de Barcelona (since 2005).
The author declares that he/she has no conflicts of interest in relation to the present Guide.
Nuria Aresté-Fosalba (narestef@senefro.org).
Degree in Medicine and Surgery (Universidad de Sevilla)(1993). Resident in training, Hospital Virgen Macarena (Sevilla)(1994-1998). Specialist in Nephrology, Hospital Virgen Macarena de Sevilla, since 2001. Supervisor of the Peritoneal Dialysis Unit from 2007 to date. Member of the Infections and Antibiotic Policy Commission of Hospital Virgen Macarena de Sevilla.
The author declares that he/she has no conflicts of interest in relation to the present Guide.
Ana Bordes-Benítez (aborben@gobiernodecanarias.org).
Section Chief of Microbiology, Hospital Universitario Dr. Negrín (Las Palmas).
The author declares that he/she has no conflicts of interest in relation to the present Guide.
María José Castro-Notario (mjcasnot@gmail.com)
Nurse specialized in Peritoneal Dialysis. Hospital Universitario la Paz (Madrid).
The author declares that he/she has no conflicts of interest in relation to the present Guide.
Antonio Fernández-Perpén (afdezperpen@senefro.org).
Degree in Medicine (Universidad Complutense, Madrid)(1992). Resident in training in Nephrology (Hospital Universitario la Princesa)(1994-97). Nephrologist, Hospital Universitario la Princesa (Madrid), since 2004. Supervisor of the Area of Peritoneal Dialysis.
The author declares that he/she has no conflicts of interest in relation to the present Guide.
María José Fernández Reyes (jfernandezre@saludcastillayleon.es)
Degree in Medicine, Universidad Autónoma de Madrid (1989). Specialist in Nephrology, Hospital Universitario la Paz 1990-1993. Doctor of Medicine, Universidad Autónoma de Madrid (2011). Specialist in Nephrology of Complejo Hospitalario de Segovia, since 1995 and Head of the Nephrology Unit since 2011. Principal investigator or co-investigator in over 100 publications in indexed journals. Collaborator in various working groups in peritoneal dialysis (Grupo Centro de Diálisis Peritoneal, Grupo de Apoyo al Desarrollo de la Diálisis Peritoneal).
The author declares that he/she has no conflicts of interest in relation to the present Guide.
Oriol Gasch-Blasi (ogasch@tauli.cat).
Degree in Medicine, Universidad de Barcelona. Specialist in infectious diseases, with professional activity at Hospital Parc Taulí (Sabadell), since 2012. Associate Professor of Medicine, Universidad Autónoma de Barcelona. Coordinator of the infectious diseases research group, with over 40 publications in indexed journals.
The author declares that he/she has no conflicts of interest in relation to the present Guide.
José Manuel Gil-Cunquero (jmgilc@senefro.org).
Nephrologist in charge of the Unit of Advanced Chronic Kidney Disease and Peritoneal Dialysis, in the Clinical Management Unit of Nephrology, Hospital Universitario de Jaén, since 2004.
The author declares that he/she has no conflicts of interest in relation to the present Guide.
Juan Carlos Julián-Mauro (Federación Alcer) (jcjulian@alcer.org).
Doctorate from Universidad Autónoma de Madrid (UAM) in Health Psychology, Department of Social Psychology and Methodology. Degree in Psychology, specialized in Occupational and Organizational Psychosociology. Postgraduate degree in management of Patient Associations, ESADE. General Director of the Federación Nacional de Asociaciones ALCER, since 2006. Collaborating Professor of the Universidad Europea de Madrid in psychosocial aspects of the renal patient, in the Expert Course on Nursing in Dialysis (2014-17). Collaborating investigator of the Faculty of Psychology of the UAM, since 2015. Currently, representative of ALCER at the International Kidney Cancer Coalition (IKCC) and European Federation of Genetic Kidney Diseases (FEDERG), and supervisor of the Action Plan of the European Kidney Patients Federation (EKPF).
The author declares that he/she has no conflicts of interest in relation to the present Guide.
José Ignacio Minguela-Pesquera (joseignacio.minguelapesquera@osakidetza.net).
Degree in Medicine (Universidad del País Vasco)(1988). Resident in training in Nephrology, Hospital de Cruces 1991-1994. Specialist in Nephrology (Hospital Txagorritxu and Hospital Universitario Araba) (1996-2020). Professional activity focused on peritoneal dialysis. Head of Department, Hospital OSI Bilbao-Basurto. Secretary of the Sociedad Norte de Nefrología during 10 years.
The author declares that he/she has no conflicts of interest in relation to the present Guide.
María Antonia Munar-Vila (mariaa.munar@ssib.es).
Specialist in Nephrology, Hospital Universitario Son Espases (Mallorca)(2001). Supervisor of the Peritoneal Dialysis Unit (2011). Professor in the Peritoneal Dialysis course (Grup de Diàlisi Peritoneal de Catalunya i Balears, of the Societat Catalana de Nefrologia)(2013).
The author declares that he/she has no conflicts of interest in relation to the present Guide.
Miguel Núñez del Moral (nmoral76@hotmail.com).
Nursing Degree, Universidad de Oviedo 1997 and University Expert in Hemodialysis 1998. Professionally dedicated to peritoneal dialysis since 2008. Member of peritoneal dialysis of the SEDEN since 2014. Associate Professor of Health Sciences, Universidad de Oviedo, since 2018. Supervisor of the Peritoneal Dialysis Unit, Hospital Universitario Central de Asturias, since 2018. Director of the Nephrology Care Research Group, Instituto de Investigación Sanitaria, Principado de Asturias (ISPA), since 2019.
The author declares that he/she has no conflicts of interest in relation to the present Guide.
Teresa Pérez-López (Teresa.Perez.Lopez@sergas.es).
Nursing Degree. Supervisor of the Hospital and Home Dialysis Unit (Hospital Universitario de A Coruña).
The author declares that he/she has no conflicts of interest in relation to the present Guide.
Jose Portolés-Pérez (josem.portoles@salud.madrid.org).
Degree from UCM Complutense (Madrid 1988). Doctorate UCM Madrid 1992. Resident in training Hospital Clínico San Carlos Madrid, 1990-1993. Head of the Department of Nephrology, Hospital Universitario Puerta de Hierro, Madrid, since April 2011. Associate Professor of the UAM, since 2011.
The author declares that he/she has no conflicts of interest in relation to the present Guide.
Pedro Quirós-Ganga (pedrol.quiros.sspa@juntadeandalucia.es).
Department of Nephrology, Hospital Universitario Puerto Real (Cádiz).
The author declares that he/she has no conflicts of interest in relation to the present Guide.
Maite Rivera-Gorrín (maiteelizabeth.rivera@salud.madrid.org)
Section Chief of Nephrology (in charge of the area of Peritoneal Dialysis) (2017). Numerous communications and publications in the field of Peritoneal Dialysis.
The author declares that he/she has no conflicts of interest in relation to the present Guide.
Carmen Rodríguez-Suarez (CARMENRS@telefonica.net).
Degree in Medicine and Surgery (Universidad de Oviedo)(1982). Resident in training in Nephrology (Hospital General de Asturias)(1983-86). Specialist in Nephrology (Hospital Central de Asturias)(1986). Professional activity focused particularly on chronic kidney disease and renal replacement therapy. Supervisor of the Peritoneal Dialysis Unit, HUCA, since its creation in 1993. Development of the “Peritoneal ultrafiltration in refractory heart failure program” since 2005.
Mario Sánchez-Camargo (Alcer Giralda)( mario.sanchez@alcergiralda.org).
The author declares that he/she has no conflicts of interest in relation to the present Guide.
María Sagrario Soriano-Cabrera (marias.soriano.sspa@juntadeandalucia.es).
Resident in training in Nephrology (Hospital Universitario Reina Sofía de Córdoba) 1995-1999. Nephrologist, Hospital Universitario Reina Sofía, since 1999. Nephrology residents tutor from 2007 to 2018. Head of the Department of Nephrology, Hospital Universitario Reina Sofía, since 2019. Doctor of Medicine, Universidad de Córdoba (2001). Associate Professor, Universidad de Córdoba, since 2004. Research Group of the Maimónides Research Institute, Hospital Universitario Reina Sofía de Córdoba and Universidad de Córdoba: Inflammation in chronic renal failure. Member of the Steering Committee of the Sociedad Española de Nefrología. Participation in the Peritoneal Dialysis Group of the Sociedad Española de Nefrología, in the Advanced Chronic Kidney Disease working group and in the Anemias Group of the Society.
The author declares that he/she has no conflicts of interest in relation to the present Guide.
Please cite this article as: Pérez Fontán M, Moreiras Plaza M, Prieto Velasco M, Quereda Rodriguez-Navarro C, Bajo Rubio MA, Borràs Sans M, et al. Guía clínica de la Sociedad Española de Nefrología para la prevención y tratamiento de la infección peritoneal en diálisis peritoneal. Nefrologia. 2022;42:1–55.
- Home
- All contents
- Publish your article
- About the journal
- Metrics
- Open access