In the present study, clinical criteria used by Spanish nephrologists when approaching chronic kidney disease (CKD) in kidney recipients, as well as their level of maintenance and control of renal function, were evaluated.
MethodsAn epidemiological, observational, multicenter, nation-wide, prospective study was carried out, with a 6-month follow-up period. Three hundred and sixty-eight adult patients with stage 3 kidney disease after a 24-month or longer post-transplantation follow-up period were included. Visits schedule included a retrospective visit, a baseline visit, an optional mid-term visit, and a final visit at month 6.
ResultsMean time since kidney transplantation was 8.2±5.4years. Most common pre-transplant cardiovascular risk factors were high blood pressure (80.2%), followed by high cholesterol levels (61.7%). Serum creatinine levels showed a statistically significant decrease from baseline visit to 6-month visit (0.06±0.22; p<.0001), and glomerular filtration rate (GFR) reduction was −1.03±6.14 (p=0.0014). Significant independent prognostic factors for GFR worsening were: higher 24-h proteinuria (OR=1.001 per mg; p=.020), longer time since transplantation (OR=1.009 per month; p=.017), and lower hemoglobin levels (OR=1.261 per g/dl; p=.038). Donor age also had some negative influence (OR=1.021 per year; p=.106). Biopsies were obtained in only 8% of kidney transplant recipients with stage 3 CKD with an intervention being carried out in 25.4% of cases.
ConclusionsSecondary markers and factors resulting in CKD progression, particularly anemia, are still frequently uncontrolled after kidney transplantation. Only about 2% of patients benefit from a therapeutic intervention based on a biopsy. Clinical perception differs from objective measures, which results in an obvious clinical inertia regarding risk factor control in such patients.
El presente estudio ha evaluado el criterio clínico que utilizan los nefrólogos españoles frente a la disfunción renal crónica (DRC) en receptores de trasplante renal (TR), y el grado de mantenimiento y control de la disfunción renal.
MétodosEstudio observacional, epidemiológico, multicéntrico, nacional y prospectivo, con un período de seguimiento de 6meses. Se incluyeron 368 pacientes adultos con disfunción renal de grado3 con un período mínimo de evolución posterior al trasplante de 24meses. La programación de las visitas incluyó una visita retrospectiva, una visita inicial, una visita intermedia opcional y una visita final al sexto mes.
ResultadosEl tiempo medio desde el TR fue de 8,2±5,4años. La hipertensión (80,2%), seguida por la hipercolesterolemia (61,7%), fueron los factores de riesgo cardiovascular previos al trasplante más frecuentes. Las concentraciones de creatinina sérica entre la visita inicial y la visita de los 6meses mostraron una diferencia estadísticamente significativa de 0,06±0,22 (p<0,0001), y la diferencia del filtrado glomerular (FG) fue de −1,03±6,14 (p=0,0014). Los factores pronósticos independientes significativos del empeoramiento del FG fueron: proteinuria a 24h más alta (OR=1,001 por cada mg; p=0,020), más tiempo desde el trasplante (OR=1,009 por cada mes; p=0,017) y concentraciones bajas de hemoglobina (OR=1,261 por cada g/dl; p=0,038). También se observó cierta influencia negativa de la edad del donante (OR=1,021 por cada año; p=0,106). Solo se realizó biopsia en el 8% de los casos de receptores de TR con DRC de grado 3, suponiendo alguna intervención en el 25,4% de los casos.
ConclusionesCon frecuencia los marcadores secundarios y los factores de progresión de la DRC siguen sin estar controlados después del TR, principalmente la anemia. Solo aproximadamente el 2% de pacientes se benefician de una intervención terapéutica basada en una biopsia. Existe una disparidad entre la percepción clínica y los parámetros objetivos, que conduce a una clara inercia clínica del control de los factores de riesgo de estos pacientes.
There has been a recent progress in immunosuppressive treatment, however long term survival for kidney transplant patients has not increased significantly over the last ten years.
Chronic kidney disease (CKD) in kidney transplant patients (KT) is a frequent complication, the treatment of which is not usually simple, since it depends, largely, on the clinical symptoms of each patient and the severity of the dysfunction. CKD is related to various factors; such as the characteristics of the transplant (donor, conservation), the specific features of the recipient, the immunosuppression treatment and the clinical outcome of the transplant.1
The most frequent causes of loss or kidney transplant failure are CKD or death of the patient with functional kidney transplant. This is, observed at a rate of 3–5% per year.2
CKD in kidney transplant is defined by a gradual deterioration of kidney function, with interstitial fibrosis and tubular atrophy that causes proteinuria, high blood pressure, and a gradual increase of serum creatinine. Mild CKD (grade I of Banff) is observed in nearly all transplants at the end of the first year of transplantation, and grade II and III, is present in 90% of patients ten years after transplant.3
Several studies in patients including biopsies performed periodically have shown that some parameters of measurement of kidney function (e.g., creatinine) underestimate the severity of CKD. Therefore, biopsies are an essential tool for an accurate diagnosis of CKD.3,4
The gradual deterioration of the kidney function is often accompanied by complications related to the presence of kidney failure (proteinuria, high blood pressure, diabetes, hyperlipidemia, anemia, metabolic acidosis, hyperphosphatemia, etc.).5–7 As it has been shown in several cohort studies of KT. Many patients with CKD have accelerated risk of deterioration of KT function as a consequence of these comorbidities.8 The adequate treatment for these complications and the prevention CKD progression require more attention by the nephrologists.8 As an example, in CKD associated tone phrotoxicity induced by calcineurin inhibitors (CNI), the possibility of reduced exposure by means of decreasing the dosage of CNI, or even discontinuation if possible, must be considered.9
The objective of this study was to assess the nephrologist's clinical approach to kidney dysfunction in kidney transplant patients subjected to maintenance treatment. Additionally, it was assessed the treatment and control of markers and factors that favors progression of kidney dysfunction, such as hypertension, urine protein and anemia, and the presence of cardiovascular risk factors in these patients.
Patients and methodsA non-interventional, multicentre, national and prospective study was carried out, with a follow-up period of six months. Initially, 446 adult kidney transplant recipients in maintenance treatment were included, between March 2009 and March 2010. Of these, 368 were ultimately included for final assessment in this study.
Patients included had to be recipients of a simple kidney transplant, adult, with CKD-3 grade 3 according to the new guidelines K/DOQI, Kidney/Disease Outcomes Quality Initiative (glomerular filtration rate, GF of 30–59ml/min), having completed a minimum period of 24 months of transplantation, and having granted their consent to participate in the study. The K/DOQI guidelines recommend an estimation of the glomerular filtration rate with the MDRD formula.10 The exclusion criteria were: presence of dual or multiorganic kidney transplant recipients, and chronic kidney disease of grade 3 (Banff scale) according to kidney graft biopsy.
Schedule of visits included a retrospective visit (between 6 and 9 months earlier), a starting visit (month 0), an optional intermediate visit and a final visit (month 6). All patients signed the informed consent to participate in the study. A Clinical Research Ethics Committee authorized the study, which was carried out according to the Declaration of Helsinki.
The information included in the present study was obtained by means of personal interviews with the patient and data collection from clinical history in 47 outpatient kidney transplant clinics from hospitals in Spain. The information of interest was: demographic data of patients and their medical history, aetiology of the terminal kidney failure (TKF), cardiovascular risk factors prior transplantation, clinical evolution of the transplant, age and sex of the donor, level of CKD analysed retrospectively, and at initial, intermediate (optional) and final visits, presence of secondary markers of CKD (proteinuria, serum creatinine level, GFR, blood pressure, haemoglobin level), blood analysis data, induction t and immunosuppression treatment at discharge.
Additionally, a detailed history was obtained after transplantation, including information about clinical data of interest after transplantation (i.e., acute rejection, diabetes, hypertension and malignancies), diagnosis of CKD, kidney biopsies performed, immunosuppression and other medications. Further, treatment of comorbidites was also recorded. During the final visit, information was gathered about the morbidity and mortality of the patient during the study period.
The objective criteria to assess the control of comorbidities was based on according to the corresponding guidelines of reference: diabetes (blood sugar level while fasting <120mg/dl)11; hypertension (blood pressure <130/85mmHg)12; mineral metabolism (Ca: 8.4–9.5mg/dl, P: 2.7–4.6mg/dl, iPTH<6.5pg/ml)13; hypercholesterolemia (LDL cholesterol<100mg/dl and HDL>40mg/dl in males and >46mg/dl in females); hypertriglyceridemia ≤200mg/dl).14 Finally, an questionnaire was given to the nephrologists to provide an opinion about the changes they made in the immunosuppression therapy so the clinical approach of the physician could be assessed.
Statistical analysisA descriptive analysis was made of the initial demographic and clinical variables of all the patients included in the study. The prevalence and the 95% confidence interval (CI) associated with the qualitative variables were calculated in the retrospective, initial, intermediate and final visits. This serve to compare frequencies and means between variables. The Kappa coefficient was used to assess the concordance between the diagnostic criteria established according to the clinical criteria and according to the objective functional criteria for the CKD markers.
Student's t test, ANOVA for repeated measurements or Wilcoxon test, was used depending on the characteristics of each variable. McNemar's test was used for comparisons of qualitative variables.
A multiple regression analysis was used to determine the factors that predict of worsening of GFR. This analysis was based on patients showing a GFR ≥10% in relation to the initial visit (n=67). In the multiple regression analysis, all the demographic and clinical variables which were close to statistical significance in prior univariate regressions (p<0.1) were included. A logistic procedure was used to extract the variability step by step, with goodness-of-fit assessment by means of the Hosmer and Lemeshow tests.
The data were analyzed using the version 9.1 or later of the statistical software SAS.
ResultsOut of the initial sample of 446 identified patients, a total of 368 patients with CKD of grade 3 (82.51%) met all the inclusion criteria and none of the exclusion criteria, and were finally included in the analysis of the present work.
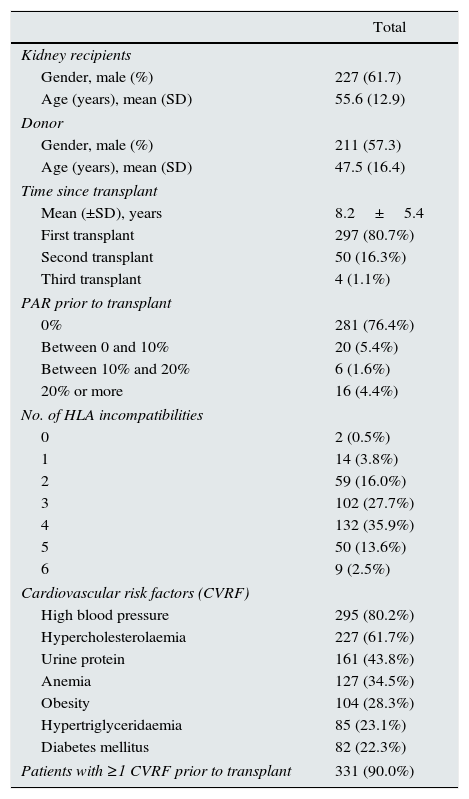
Demographic and descriptive dataMean age of patients was 55.6±12.9 years and 61.7% were males (Table 1). The average time since kidney transplant was 8.2±5.4 years. The most frequent case of terminal kidney failure (TKF) was chronic glomerulonephritis, which was observed in 114 cases (31.0%), whereas only one patient (0.3%) was found to have nephrotoxicity. Hypertension (80.2%) followed by anemia (34.5%) were the most frequent cardiovascular risk factors before transplantation; other risk factors were dyslipidemia (22.8%) and prior cardiovascular disease (3.5%).
Initial characteristics of the sample.
| Total | |
|---|---|
| Kidney recipients | |
| Gender, male (%) | 227 (61.7) |
| Age (years), mean (SD) | 55.6 (12.9) |
| Donor | |
| Gender, male (%) | 211 (57.3) |
| Age (years), mean (SD) | 47.5 (16.4) |
| Time since transplant | |
| Mean (±SD), years | 8.2±5.4 |
| First transplant | 297 (80.7%) |
| Second transplant | 50 (16.3%) |
| Third transplant | 4 (1.1%) |
| PAR prior to transplant | |
| 0% | 281 (76.4%) |
| Between 0 and 10% | 20 (5.4%) |
| Between 10% and 20% | 6 (1.6%) |
| 20% or more | 16 (4.4%) |
| No. of HLA incompatibilities | |
| 0 | 2 (0.5%) |
| 1 | 14 (3.8%) |
| 2 | 59 (16.0%) |
| 3 | 102 (27.7%) |
| 4 | 132 (35.9%) |
| 5 | 50 (13.6%) |
| 6 | 9 (2.5%) |
| Cardiovascular risk factors (CVRF) | |
| High blood pressure | 295 (80.2%) |
| Hypercholesterolaemia | 227 (61.7%) |
| Urine protein | 161 (43.8%) |
| Anemia | 127 (34.5%) |
| Obesity | 104 (28.3%) |
| Hypertriglyceridaemia | 85 (23.1%) |
| Diabetes mellitus | 82 (22.3%) |
| Patients with ≥1 CVRF prior to transplant | 331 (90.0%) |
Abbreviations: SD: standard deviation, PRA: profile of reactive antibodies; HLA: Human Leukocyte Antigen.
The initial serum creatinine concentration and the glomerular filtrate (GFR) were 1.6±0.3mg/l and 43.6±7.6ml/m, respectively. At the time of inclusion, 24h urine protein collected was 425.7±639.9mg (25.5% of patients had more than 300mg), 39.7% of patients presented anemia, 89.7% hypertension and 26.1% diabetes.
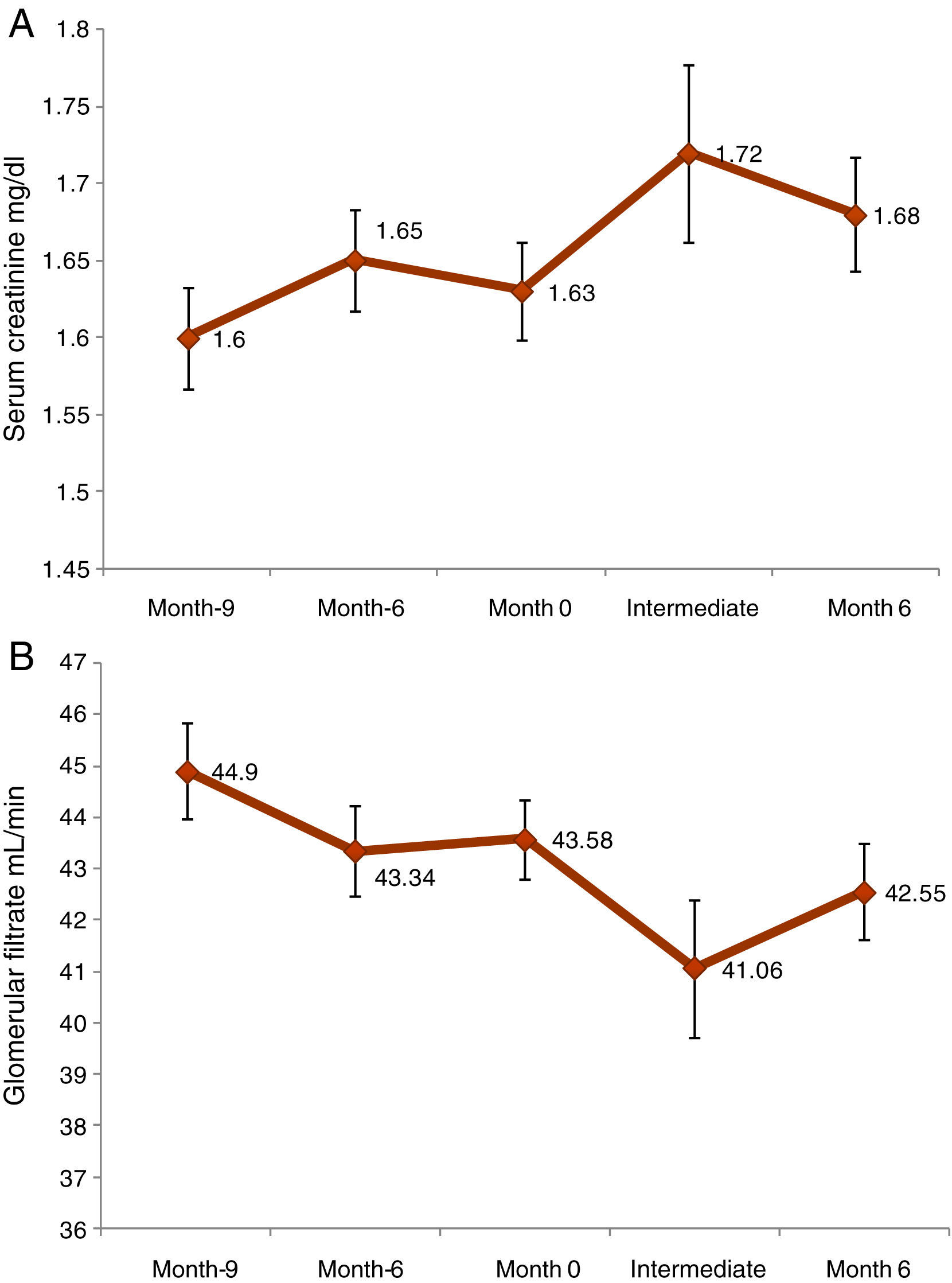
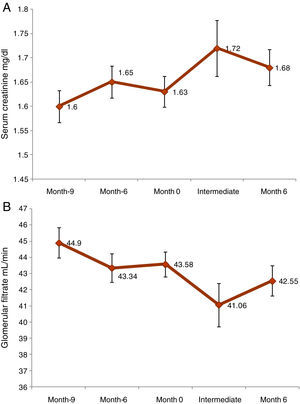
A statistically significant increase in serum creatinine (0.06±0.22mg/dl, p<0.0001) was observed from the initial to month 6 visit. In the case of GFR, the difference was −1.03±6.14ml/min (p=0.0014) (Fig. 1). From initial visit and month 6 a reduction of GFR of more than 18% was observed in18.2% of patients. Nephropathy from BK virus was investigated in 32.6% of patients.
Neoplasia was diagnosed in 9.8% (n=36) of transplanted patients and the immunosuppression therapy was modified in 26 patients (72.2%). A.14.7% of patients (n=54) experienced cardiovascular complications: most frequent complication was angina (27.8%),, peripheral arterial disease was observed in 22.2%.
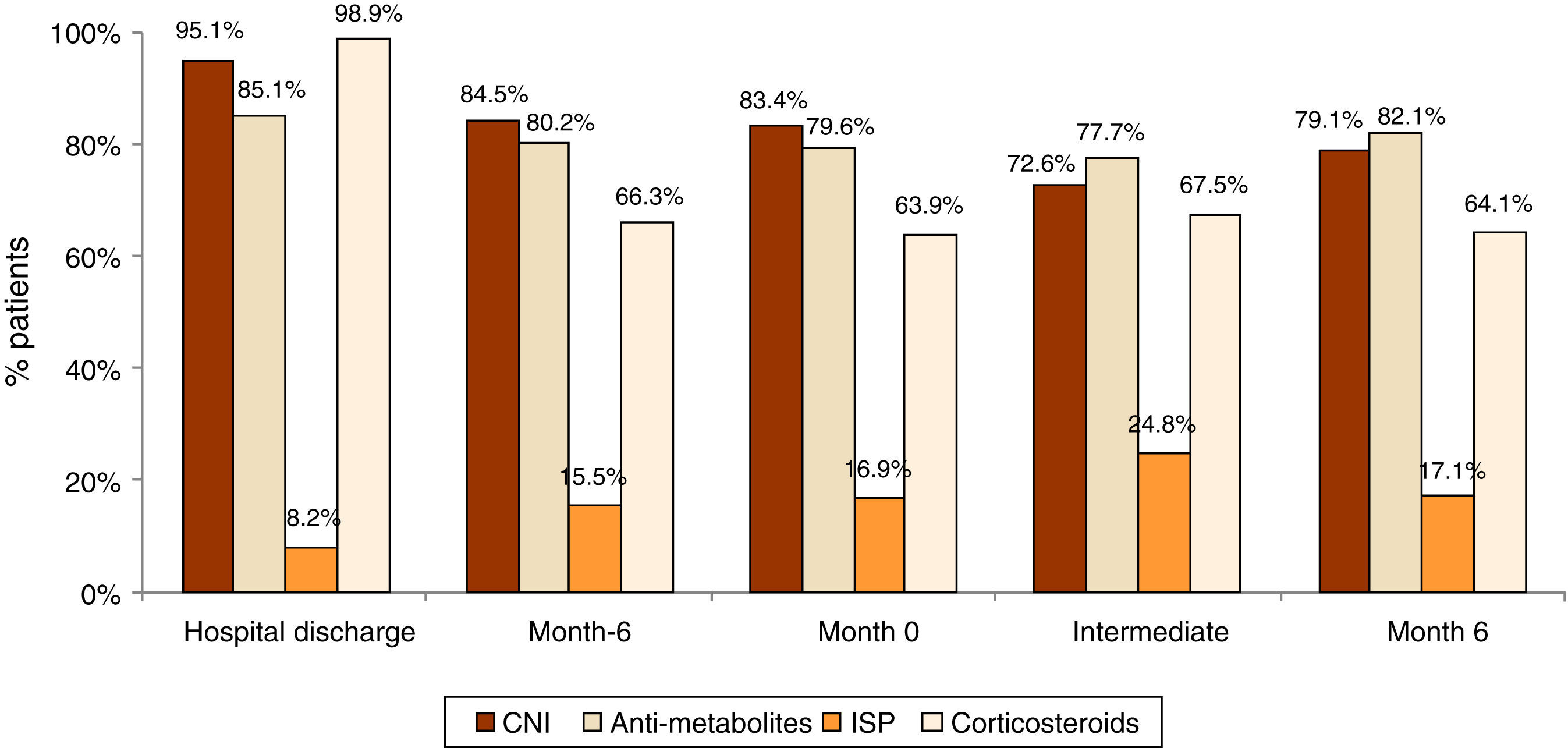
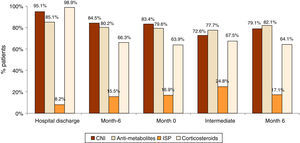
Immunosuppression treatment and biopsiesTreatment for proteinuria, anemia and hypertension was set in 34.2%, 34.0% and 86.4% of patients and the treatment was strengthened in 60–73% of the uncontrolled patients. Despite treatment, at month 6, 26.1%, 7.3% and 64.7% of patients did not meet the treatment objectives for proteinuria, anemia and hypertension respectively. During follow-up, no significant changes were observed in the treatment for immunosuppression (Fig. 2). The most frequently used immunosuppression drug was calcineurin inhibitors (CNI) combined with anti-metabolites and esteroids.
A biopsy was performed after transplantion in 28 patients (7.6%), and the most frequent finding was chronic rejection (n=8). The biopsy led to modification of therapy in 25.4% of cases; change in immunosuppression was the most frequent intervention (50.0%). In 83.3% of patients kidney function stabilize after modification of treatment. The biopsy was prescribed following clinical criteria in all cases, and according to the opinion of the physicians the biopsy was useful in 88.9% of cases. Physicians also considered that the techniques of immunohistochemistry and immunofluorescence were very useful to decide therapeutic strategies directed to stabilise kidney function (88.9% of cases).
Clinical approach of doctorsThe nephrologist questionnaire shows that 88.1% of doctors used the level of proteinuria as a CKD marker, and more than 58% used anemia and hypertension in the same way. In addition, 80.6% of doctors calculated the change of GFR during the last year to assess the kidney function deterioration. Doctors have identified hypertension, dyslipidaemia, and hyperglycaemia as equally and clinically adequate factors to assess the evolution of CKD (91.7%; 91.7% and 88.9%; respectively). In the case of hypertension, most patients had a reduction of anti-calcineurin and an increase in mycophenolate (58.3%) with reduction or discontinuation of esteroids (both 66.7%). In the case of hyperglycaemia, the priority change was the reduction of treatment with esteroids (88.3%) or its interruption (80.6%). Finally, in the case of dyslipidaemia, the priority change was the reduction of treatment with esteroids (83.3%), and its interruption in 83.3% of cases.
A 80.6% of the doctors considered that the kidney transplant recipients with CKD required a more intense control of cardiovascular risk factors, with blood more frequent biochemistries and echocardiograms.
With regard to the control of secondary markers of CKD, there were no significant differences in hypertension (p=0.513), nor in the urine protein rates or of urine protein 24h (p=0.879) between patients during the visits.
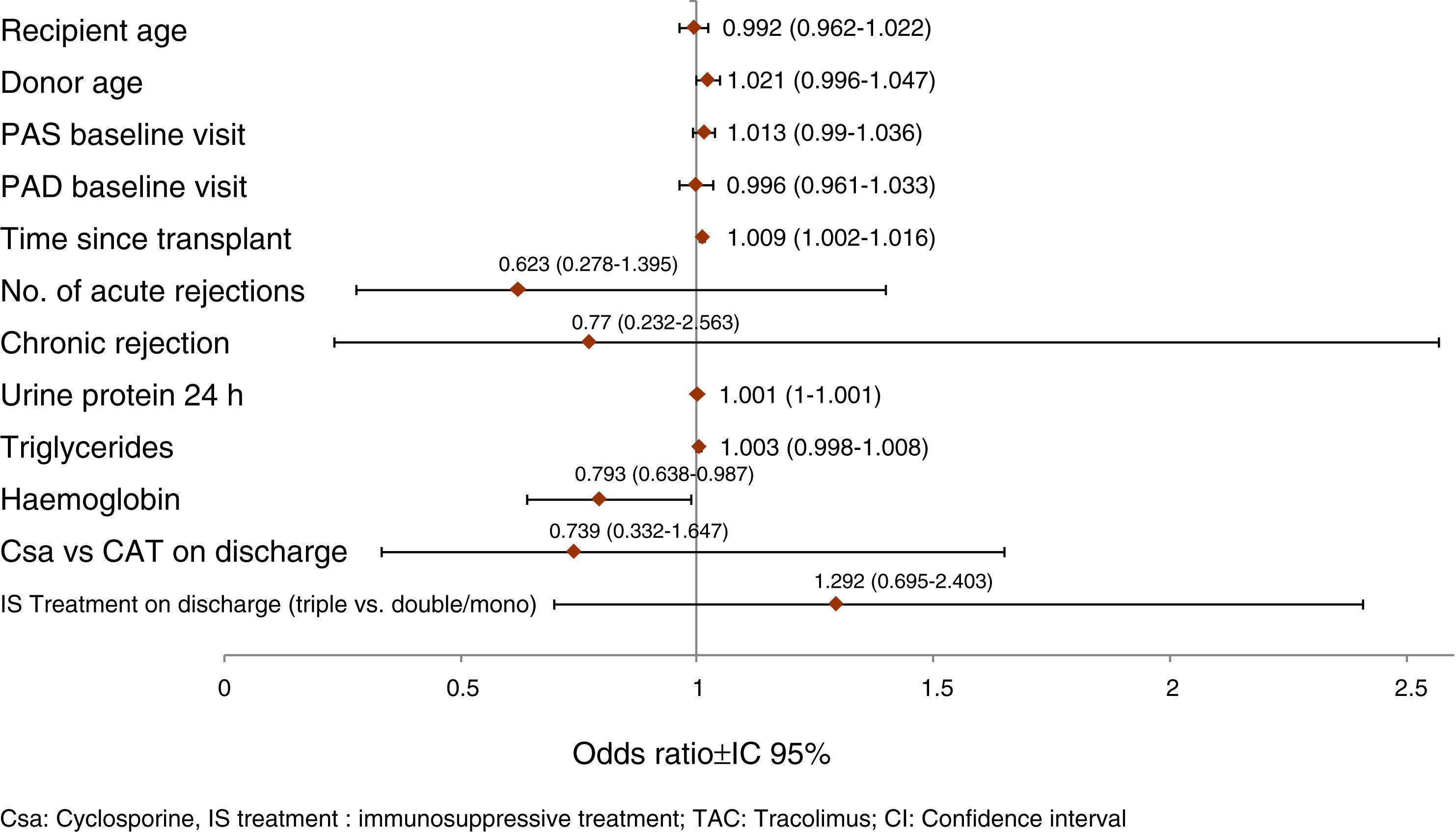
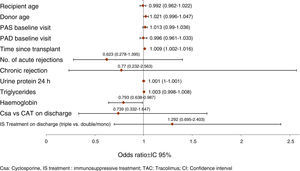
Prognostic factors of GF worseningThe model of multivariate logistic regression (Fig. 3) showed that the significant independent prognostic factors of GFR worsening were: 24h proteinuria (OR=1.001 per each mg, p=0.020), period of time elapsed since transplantation (OR=1.009 per each month, p=0.017) and low haemoglobin (Hb)evel (OR=1.261 per each g/dl, p=0.038). Also, a negative influence of the donor's age was observed (OR=1.021 per each year, p=0.106).
Management of CKDIn the initial visit, approximately one third of the patients had an appropriate management of proteinuria, 89.3% in had controlled hypertension, and only 37.7% received optimal treatment of anemia. Physicians failed to treat proteinuria, hypertension and anemiain 31.3%, 18.6% and 16.7% of the cases respectively.
In the initial visit, there was a 27.3% of patients with good control of mineral metabolism, 20.3% with acceptable control of diabetes and 72.9% with controlled cholesterol. In the final visit at month 6, only 16 patients had no appropriate control of mineral metabolism parameters; 37.5% of patients and an intensified control of these parameters. In the case of diabetes and hypercholesterolemia, there was an intensification of control in approximately 65% of the previously uncontrolled patients.
The cardiovascular risk factors such as glucose, glycosylated haemoglobin, blood sugar levels, HDL cholesterol, LDL cholesterol, total cholesterol and triglycerides, remained stable throughout all the visits.
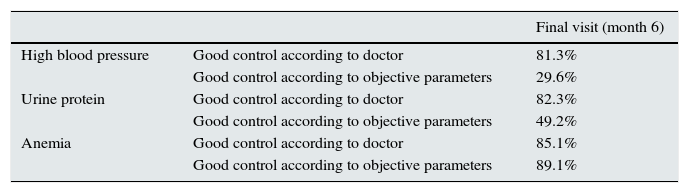
There is disparity between the clinical perception and the objective parameters, which leads to a clear clinical inertia with respect to control of associated risk factors, hypertension and proteinuria (Table 2). Regarding hypertension, the perception of good control by the doctor was greater than the actual objective parameters gathered from the patient sheet (81.3% compared to 29.7%). Therefore, the Kappa coefficient of concordance was quite low and non-significant, with values between 0.0762 in the initial visit and 0.1438 in the final visit.
Degree of control of risk factors of kidney dysfunction (hypertension, urine protein and anemia) in the final visit.
| Final visit (month 6) | ||
|---|---|---|
| High blood pressure | Good control according to doctor | 81.3% |
| Good control according to objective parameters | 29.6% | |
| Urine protein | Good control according to doctor | 82.3% |
| Good control according to objective parameters | 49.2% | |
| Anemia | Good control according to doctor | 85.1% |
| Good control according to objective parameters | 89.1% |
Good control according to objective parameters or confirmed: PAS/PAD<130/80mmHg; urine protein 24h ≤300mg/24h; hemoglobin>11g/dl.
The perception of good control of proteinuria by doctors was greater (82.3%) than the objective parameters (49.2%). In this case, the coincidence between clinical perception and the objective parameters was greater than in hypertension, with a Kappa coefficient close to 0.5 in all visits.
Finally, the clinical perception of good control of anemia corresponded almost completely with the objective criteria (85.1% compared to 89.1%); the coincidence increased between the retrospective and the final visits.
A 15–32% of patients who needed initial treatment for hypertension and proteinuria were not objectively controlled.
All patients were subjected to changes in their treatment and to another visit after the transplant. The main reason for changes in the treatment was the presence of comorbidities (12.5%). Different specialists were consulted in 14.1% of cases, mostly endocrinologists. An additional unforeseen visit was scheduled in25.8% of patients. In the final visit, a Doppler ultrasound of the transplanted kidney was performed in 4 patients, and there were 13 transplant obtained.
DiscussionWe have carried out a non-interventional, prospective study to assess the clinical approach of doctors in the treatment of patients with CKD maintained with a kidney transplant in Spain.
Our main results show that hypertension and anemia are the most frequent cardiovascular risk factors observed before transplantation. We also show that there are secondary markers of CKD that cannot be controlled after transplantation, mainly anemia, which persists without adequate treatment. Actually, our data shows that there is a 16.7% of patients without intensified treatment for anemia and this represents a considerable clinical inertia with major implications in the progression of CKD, with a low initial rate of adequate treatment of anemia (37.7%). This situation is similar to that observed in recent prospective studies, showing that the control of haemoglobin values reduces the progression of chronic kidney disease in allotransplant KT patients.15,16
A biopsy was obtained only in a limited number of cases of KT recipients with CKD grade 3, this is in contrast with recent data published suggesting the need for biopsies in these type of.3,4,17 The biopsy results led to therapeutic intervention in only 25.4% of patients who underwent this procedure, a percentage which may be considered low according to recent recommendations.17
Several studies evaluating results from kidney biopsies have shown that the use of serum creatinine concentration for the diagnosis of nephropathy may lead to underestimation of the severity of CKD.18 According to some authors, biopsy is a procedure recommended in patients with serum creatinine concentrations increased by more than 20% of the minimum creatinine concentration during the last 3–6 months, regardless of the presence of proteinuria.19
In our study, a graft biopsy was performed in 7.6% of patients between the initial and final visit, and the most frequent cause was chronic rejection. Some studies have found an acute subclinical rejection in the evaluation of pre-scheduled biopsies in patients with CKD in early stages after transplantation. This finding predicts a lower survival of the transplant.20 A recent controlled and randomised study showed that early treatment for rejection improved the clinical outcome of these patients.21 Therefore, a more precise knowledge of the causes of CKD is required so an early diagnosis and treatment can be applied. Therefore, biopsies should perform before the patient evolves to advanced CKD.
Some results conclude that an early biopsy that allows microscopic evaluation and appropriate changes in immunosuppressive treatment may be helpful in protecting the graft function.21
The recent cross-sectional ICEBERG22 study has shown that the prevalence of CKD in kidney transplant recipients is ranges from 35 and 55% depending on the diagnostic method, clinical or objective criteria such as serum creatinine or GFR. This study has also shown that CKD is a usually under-diagnosed pathology in maintenance KT recipients. Doctors only detect CKD in 4 out of the 10 patients that were diagnosed objectively. Further, the results of OBSERVA confirm that due to this under-diagnosis, most transplanted patients do not receive sufficient treatment for comorbidities. Therefore, regarding patients treatment there are considerable differences between the clinical perceptions and the objective parameters and this leads to significant clinical inertia. Avoiding this clinical inertia would be crucial in preserving kidney transplantfunction by modifying the CNI if needed, performing timely biopsy, adaptation of the immunosuppression therapy, treatment to the CKD, control of comorbidities, and the addition of recommendations for a healthy life style.23
Further, we have observed that there is a large percentage of patients with uncontrolled hypertension, which may contribute to the increase of cardiovascular comorbidity and comortality of the transplant recipients. More intense treatment is required to improve the survival of the transplant and the patients. According to current recommendations at the time of the study, the optimum control of hypertension would be a blood pressure <130/80mmHg or <125/75 in patients with urine protein.24 These authors suggest a strict control of hypertension to avoid clinical inertia in the kidney transplant centers. Other authors also suggest that reduced GF does not represent the total risk of presenting CKD, which suggests that there are other factors, such as asymptomatic cardiopathy, which could be involved in the gradual worsening of kidney function and in the transplant failure.25 Likewise, recent studies carried out in the US have stressed the fact that there is still an opportunity to improve the treatment and control of traditional factors of cardiovascular risk in kidney transplant recipients, as suggested by the high rates of uncontrolled hypertension in these patients.26,27
Recent studies on kidney transplant recipients have shown that there is poor control of cardiovascular risk factors. In these studies, large differences have been observed in the treatment of kidney transplant and non-transplanted patients with the same stage of CKD.28,29 As compared with non-transplanted CKD patients, kidney transplant recipients show a poor control of blood pressure, lipids, and haemoglobin concentration. This data suggests that an adequate control of these parameters is not achieved in the outpatient KT.
The KDIGO guidelines also provide suggestions for the effective control of serum creatinine and proteinuria.30,31 The level of serum creatinine one year after transplantion predicts poor outcome and may help determine the frequency of visits in long term care. Proteinuria has been associated with cardiovascular complications and mortality in KT patients. Therefore, the proteinuria measurement is recommended one month after transplantation as an initial value, and, every 3 months during the first year, and annually thereafter.32 It is further recommended to treat proteinuria with renin–angiotensin inhibitors in an attempt to reduce chronic kidney disease.33
Our study has several strong points, such as the large number of patients and participating centres, which represents the population of kidney transplant patients in Spain, and it reflect the treatment of these patients in every day clinical practice. Our study also has limitations, such as the short follow-up period after the kidney transplant, only 6 months. However, in patients with CKD grade 3, a follow-up of 6 months should be enough to allow the result of specific medical interventions directed to reduce worsening kidney function. Further, among the limitations, it should be noted that among the causes for progression of kidney failure, immunologic damage was not included, although it is not the objective of this study, which attempts to assess the clinical inertia in pathologies where action is still possible by following the guidelines available.
In short, besides the treatment of proteinuria with renin–angiotensin blockers, it is necessary a more rigorous control other CKD markers, with assessment of biopsies, long term follow-up, and specific strategies for the control of comorbidity factors, to improve the clinical outcome and the survival of maintenance kidney transplant recipients.34
Conflict of interestThe authors declare no conflict of interest.
Please cite this article as: Campistol JM, Gutiérrez-Dalmau A, Crespo J, Saval N, Grinyó JM. Actitud clínica frente a la disfunción renal en receptores de un trasplante renal en España. Nefrologia. 2015;35:256–263.