Actually, there are few data about glomerular filtration rate (eGFR) drop in patients with resistant hypertension and how different therapies can modify chronic kidney disease progression (CKD).
ObjectiveTo evaluate CKD progression in patients with resistant hypertension undergoing two different therapies: treatment with spironolactone or furosemide.
MethodsWe included 30 patients (21M, 9W) with a mean age of 66.3±9.1 years, eGFR 55.8±16.5ml/min/1.73m², SBP 162.8±8.2 and DBP 90.2±6.2mmHg: 15 patients received spironolactone and 15 furosemide and we followed up them a median of 32 months (28–41).
ResultsThe mean annual eGFR decrease was −2.8±5.4ml / min / 1.73m². In spironolactone group was −2.1±4.8ml / min / 1.73m² and in furosemide group was −3.2±5.6ml / min / 1.73m², p<0.01. In patients received spironolactone, SBP decreased 23±9mmHg and in furosemide group decreased 16±3mmHg, p<0, 01. DBP decreased 10±8mmHg and 6±2mmHg, respectively (p<0.01). Treatment with spironolactone reduced albuminuria from a serum albumin/creatine ratio of 210 (121–385) mg / g to 65 (45–120) mg/ g at the end of follow-up, p<0.01. There were no significant changes in the albumin / creatinine ratio in the furosemide group. The slower drop in kidney function was associated with lower SBP (p=0.04), higher GFR (p=0.01), lower albuminuria (p=0.01), not diabetes mellitus (p=0, 01) and treatment with spironolactone (p=0.02). Treatment with spironolactone (OR 2.13 IC 1.89–2.29) and lower albuminuria (OR 0.98 CI 0.97–0.99) maintain their independent predictive power in a multivariate model.
ConclusionTreatment with spironolactone is more effective reducing BP and albuminuria in patients with resistant hypertension compared with furosemide and it is associated with a slower progression of CKD in the long term follow up.
En la actualidad, existen pocos datos sobre la evolución de la función renal en pacientes con HTA resistente y enfermedad renal crónica (ERC), así como de la influencia de diferentes tipos de tratamiento en dicha progresión.
ObjetivoEvaluar la progresión de la ERC en pacientes con ERC e hipertensión arterial resistente sometidos a dos estrategias terapéuticas diferentes: tratamiento con espironolactona versus furosemida.
MétodosIncluimos 30 pacientes 21 H, 9M con una edad media de 66,3±9,1 años, FGe 55,8±16,5ml/min/1,73m², PAS 162,8±8,2 y PAD 90,2±6,2mmHg: 15 tratados con espironolactona y 15 con furosemida seguidos durante un tiempo medio de 32 meses (28–41).
ResultadosEl descenso medio anual del FGe fue de -2,8±5,4ml/min/1,73m². En el grupo de espironolactona fue de -2,1±4,8ml/min/1,73m² y en el de furosemida -3,2±5,6ml/min/1,73m², p<0,01. En los pacientes con espironolactona la PAS disminuyó 23±9mmHg vs 16±3mmHg en el grupo de furosemida (p<0,01). La PAD se redujo 10±8mmHg y 6±2mmHg respectivamente (p<0,01). El tratamiento con espironolactona redujo la albuminuria de una mediana de 210 (121–385) mg/g a 65 (45–120) mg/g al final del seguimiento, p<0,01. En el grupo de furosemida la albuminuria no descendió. La progresión más lenta en la enfermedad renal se asoció con una menor PAS (p=0,04), mayor FGe basal (p=0,01), menor albuminuria(p=0,01), no tener diabetes mellitus (p=0,01) y recibir tratamiento con espironolactona (p=0,02). El tratamiento con espironolactona (OR 2,13 IC 1,89–2,29) y la menor albuminuria (OR 0,98 IC 0,97–0,99) mantienen su poder predictivo independiente en un modelo multivariante.
ConclusionesEl tratamiento con espironolactona reduce más la PA y la albuminuria en pacientes con HTA resistente comparado con la furosemida y esto se asocia con una progresión más lenta de la ERC a largo plazo.
There is a strong association between chronic kidney disease (CKD) and resistant hypertension (HTN)1,2 and several studies have shown association between HTN and the progression of CKD.3,4 However there is little information on the decrease in the estimated glomerular filtration rate (eGFR) in patients with resistant HTN and there is even less data on the effect of therapeutic strategies to control of blood pressure (BP) on the progression of CKD of these patients with resistant HTN. In a previous study published in 2015, we observed that spironolactone was more effective than furosemide in the control of BP and albuminuria in patients with resistant hypertension and CKD; this was in an initial short-term follow-up of 6 months.5 The main results of that study were: 1) spironolactone reduced systolic (SBP) and diastolic (DBP) blood pressure more effectively than furosemide (SBP: 24±9.2mmHg vs. 13.8±2.8mmHg and DBP: 11±8.1mmHg vs. 5.2±2.2mmHg); 2) Spironolactone treatment reduced albuminuria (from 173±268mg /g to 14±24mg / g), while no significant changes in albuminuria were observed in the furosemide group, and 3) eGFR remained stable in the 2 groups of treatment.
The present study shows the “post hoc” results on the progression of CKD of an additional long-term follow-up of 3 years in these patients with resistant HTN treated with the 2 optimization guidelines of antihypertensive treatment (diuretic intensification with loop diuretics versus aldosterone blockade).
Thus, the main objective of this study is to evaluate the progression of CKD in patients with CKD and resistant HTN treated by 2 different therapeutic strategies: spironolactone vs. furosemide. Secondary objectives were to evaluate the long-term effect of these 2 therapeutic strategies in the control of BP and albuminuria.
Material and methodsStudy designThe design of the previous study is described in the original publication.5 It is a prospective, non-randomized observational study; summarizing, 30 patients with resistant HTN from the outpatient Nephrology clinic with eGFR > 30ml / min / 1.73m2 with resistant HTN defined as a BP≥130/80mmHg recorded by ambulatory BP monitoring (ABPM), despite adherence to treatment with 3 full-dose antihypertensive drugs including a diuretic. Patients were recruited during the period January 2013 to December 2013. Patients excluded had : 1) secondary causes of HTN, such as renal artery stenosis, obstructive sleep apnea syndrome or hormonal alterations; 2) eGFR < 30ml / min per 1.73m 2 calculated using the CKD-EPI formula; 3) basal potassium levels > 5 mEq / l; 4) pregnancy or breastfeeding and the non-use of any effective contraceptive method in women of childbearing age; 5) known hypersensitivity to spironolactone or furosemide, and 6) non-stable patients or hospitalized during the 3 months before baseline determinations. The sample size was calculated based on studies on the effect of diuretic treatment in the control of BP in patients with resistant hypertension. The data utilized was from: 1) the ASCOT study6 in which spironolactone was added to the antihypertensive treatment in patients with resistant HTN and the BP decreased an average of 21.9 / 9.5mmHg, and 2) data from our group,7 in which the effect of the increase in the dose of furosemide was studied in patients with HTN secondary to volume expansion and CKD, where the SBP was reduced by an average of 21.4±7.1mmHg. Accepting a 5% alpha risk, a 20% beta risk in a bilateral contrast and estimating a 10% loss, 30 subjects were required to detect a difference equal to or greater than 10mmHg in PAS. It was assumed a standard deviation (SD) of 10mmHg and a correlation coefficient between the initial and final measurement of 0.2.
The study was conducted in accordance with the principles of the Declaration of Helsinki and informed consent was obtained from all patients. Initially, the baseline treatment of patients with resistant HTN was adjusted using the usual clinical practice criteria of the responsible physician, which was based on the reference guidelines of the main scientific societies for the treatment of HTN.8–10 After adjustment of antihypertensive treatment, if the target BP was not achieved within 2 months, furosemide 40mg per day or spironolactone 25mg per day was added to improve BP control. Both the initial diagnosis of resistant HTN and follow-up (every 6 months) after treatment adjustment, was performed using ABPM. The new medication was assigned according to clinical criteria following the guidelines of good clinical practice. Fifteen patients were assigned to furosemide and 15 to the spironolactone group. Routine monitoring visits were performed at baseline, 1st and 3rd month, and then every 6 months. Demographic variables such as age, sex and cardiovascular risk factors (diabetes mellitus [DM], dyslipidemia and the history of cardiovascular disease) were analyzed. Blood samples and 24-h urine samples (albuminuria and urinary excretion of urea, creatinine, sodium and potassium) were obtained at each visit after an overnight fasting. Patients were instructed to perform 24-h urine collection properly: the patient discards his first urine on the morning of the day of collection and then begins collecting urine samples in a plastic container all day, including the first morning urine the next day. The biochemical parameters were measured using routine methods. The eGFR was calculated using the formula CKD-EPI.
The 24-h ABPM was used to increase the accuracy of BP determinations; a validated device was used at the beginning of the study and subsequently for follow-up every 6 months. The arm validated model with oscillometric measurement mode Watch BP O3, MICROLIFE was used. The average BP during the day was calculated from the values measured between 9.00 and 21.00; the nocturnal BP and the average of the BP from the values measured between 1.00 and 6.00, and the average of 24h BP was calculated from all the registered values of ABPM. The normal circadian profile is characterized by a decrease of 10–20% of the values for nocturnal BP compared to the values during daytime or activity BP (dipper profile). The absence of a decrease in nocturnal BP levels <10% was considered a non-dipper pattern. The riser pattern was considered if the average of nocturnal BP values was higher than the average of daytime.
Adverse events related to the medication were assessed at each follow-up visit. The criteria for the finalize the study and the suspension of the medication were symptomatic hypotension, serum potassium levels > 6 mEq / l, development of acute renal failure defined as an increase in serum creatinine from baseline of at least 44.2mmol / l (0.5mg / dl), a decrease in creatinine clearance of at least 50% or the need for renal replacement therapy, and any adverse side effects related and attributable to the medication administered, or at the patient's request. We defined mild hyperkalemia as serum potassium between 5.0–5.5 mEq / L and in these cases, patients received treatment with cation exchange resins, and recommendation of a potassium restricted diet.
Follow-upOnce the previous study was completed, the patients were followed for a median period of time of 32 months (28–41).
Visits and baseline data were obtained at the time of inclusion, first month and then every 6 months. Renal function, eGFR, was assessed according the CKD-EPI formula. The albumin / creatinine ratio was measured in urine collected early in the morning using an immunonephelometricmethod.
We analyze the progression of renal insufficiency based on the average annual change in eGFR. Depending on whether the annual decrease in eGFR was above or below the average, patients were separated into 2 groups. Group A: patients with rapid progression of CKD (above average); group B: patients with slow progression of CKD (below average).
The BP was measured every 6 months using ABPM. The mean values of SBP and DBP from the 24h recording were used for the analysis.
Statistical analysisValues are expressed as mean±SD or median (interquartile range) if the variables did not follow a normal distribution. The Kolmogorov-Smirnov test was used to assess the normal distribution of the values. Differences between groups of patients was analyzed using χ2 test for qualitative variables and the Student t-test or the Mann-Whitney test for quantitative variables with a normal or non-Gaussian distribution, respectively. We assume a statistically significant difference if p<0.05.
Generalized linear models were made to compare the decrease in SBP, eGFR and urine albumin / creatinine ratio between the 2 treatment groups adjusted for the baseline characteristics of BP, eGFR, albumin/creatinine ration and potassium levels.
The variables that influenced the annual change in eGFR were analyzed by multivariable linear regression. The prognostic power of predictive factors for rapid progression of CKD was assessed using a multivariable logistic regression model. We introduce in the model all covariates that in the univariable model predicted rapid progression with a p<0.01. The regression coefficients and their SD were calculated with a statistical package SPSS 16.0 (Chicago, IL, USA).
ResultsThe mean age of the 30 patients included was 66.3±9.1 years, 70% of the patients were male and 56.7% were diabetic. The average of eGFR was 55.8±16.5ml / min per 1.73m2. The mean 24h BP measured by ABPM at the baseline was 162±8/90±6mmHg and the average number of antihypertensive drugs that they received was 4.1±0.9 per patient.
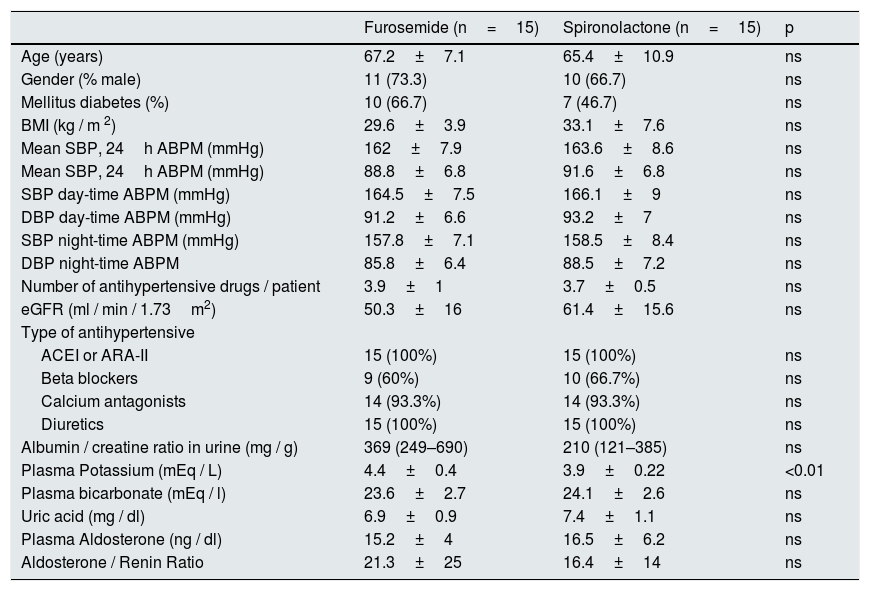
Baseline characteristics of the patients included in the spironolactone and furosemide group were described in the previous study and are included in the Table 1.
Baseline characteristics of patients in the spironolactone and furosemide group.
| Furosemide (n=15) | Spironolactone (n=15) | p | |
|---|---|---|---|
| Age (years) | 67.2±7.1 | 65.4±10.9 | ns |
| Gender (% male) | 11 (73.3) | 10 (66.7) | ns |
| Mellitus diabetes (%) | 10 (66.7) | 7 (46.7) | ns |
| BMI (kg / m 2) | 29.6±3.9 | 33.1±7.6 | ns |
| Mean SBP, 24h ABPM (mmHg) | 162±7.9 | 163.6±8.6 | ns |
| Mean SBP, 24h ABPM (mmHg) | 88.8±6.8 | 91.6±6.8 | ns |
| SBP day-time ABPM (mmHg) | 164.5±7.5 | 166.1±9 | ns |
| DBP day-time ABPM (mmHg) | 91.2±6.6 | 93.2±7 | ns |
| SBP night-time ABPM (mmHg) | 157.8±7.1 | 158.5±8.4 | ns |
| DBP night-time ABPM | 85.8±6.4 | 88.5±7.2 | ns |
| Number of antihypertensive drugs / patient | 3.9±1 | 3.7±0.5 | ns |
| eGFR (ml / min / 1.73m2) | 50.3±16 | 61.4±15.6 | ns |
| Type of antihypertensive | |||
| ACEI or ARA-II | 15 (100%) | 15 (100%) | ns |
| Beta blockers | 9 (60%) | 10 (66.7%) | ns |
| Calcium antagonists | 14 (93.3%) | 14 (93.3%) | ns |
| Diuretics | 15 (100%) | 15 (100%) | ns |
| Albumin / creatine ratio in urine (mg / g) | 369 (249–690) | 210 (121–385) | ns |
| Plasma Potassium (mEq / L) | 4.4±0.4 | 3.9±0.22 | <0.01 |
| Plasma bicarbonate (mEq / l) | 23.6±2.7 | 24.1±2.6 | ns |
| Uric acid (mg / dl) | 6.9±0.9 | 7.4±1.1 | ns |
| Plasma Aldosterone (ng / dl) | 15.2±4 | 16.5±6.2 | ns |
| Aldosterone / Renin Ratio | 21.3±25 | 16.4±14 | ns |
ARA-II: angiotensin II receptor antagonist; eGFR: estimated glomerular filtration; ACEI: angiotensin converting enzyme inhibitors; BMI: body mass index; ABPM: ambulatory blood pressure monitoring; ns: not significant; DBP: diastolic blood pressure; SBP: systolic blood pressure.
The median follow-up of the patients was 32 months (28–41) and none of the patients was lost during the follow-up.
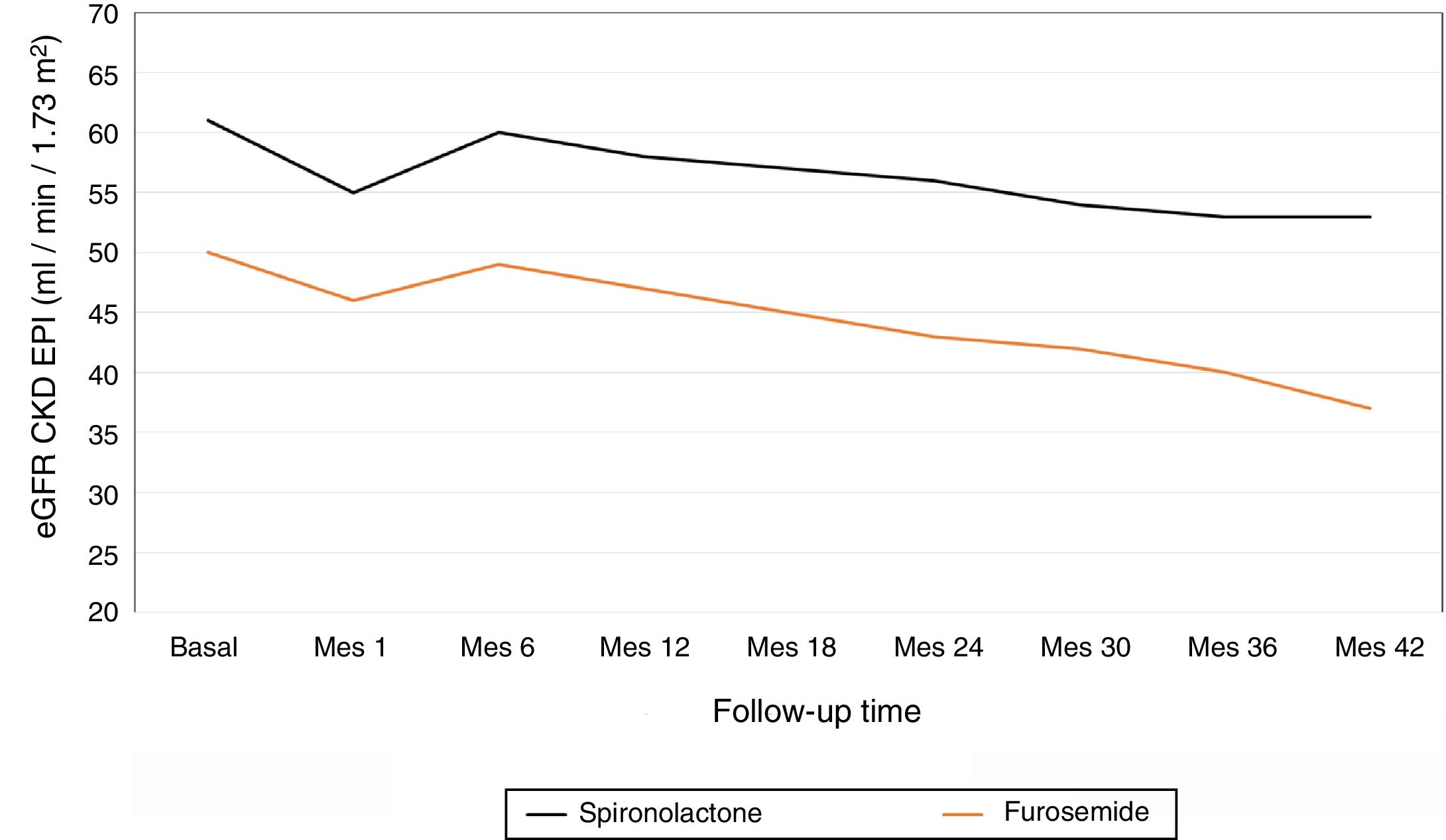
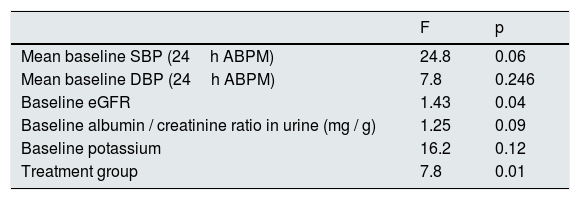
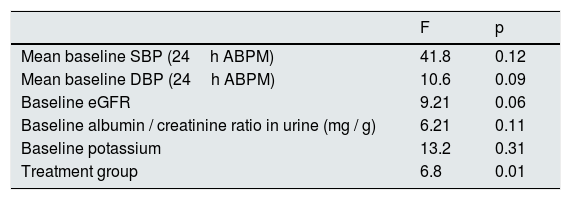
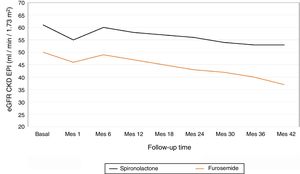
The average annual decrease in eGFR in the total number of patients was –2.8±5.4ml / min / 1.73m2. Fig. 1 depicts the evolution of eGFR in patients receiving spironolactone or furosemide. In the group of patients who received spironolactone the decline in eGFR was it was –2.1±4.8ml / min / 1.73m2 and in those receiving furosemide –3.2±5.6ml / min / 1.73m2. A general linear model was performed to compare the decrease in eGFR in the 2 treatment groups adjusted for baseline values of SBP, DBP, eGFR, albumin / creatinine index and potassium levels. The factors that resulted to be independently associated with the decrease in eGFR were the baseline level eGFR (p=0.04) and the treatment received (p=0.01) (Table 2). A more marked reduction in eGFR was observed during the first month of the follow-up in patients from both groups, (eGFR –4.9ml / min / 1.73m2 in all patients, –5.8ml / min / 1.73m2 in the spironolactone group and –4.1ml / min / 1.73m2 in the furosemide group). After this initial decrease, the eGFR it was partially recovered at 6 months of follow-up.
General linear model for factors associated with the decrease in eGFR.
| F | p | |
|---|---|---|
| Mean baseline SBP (24h ABPM) | 24.8 | 0.06 |
| Mean baseline DBP (24h ABPM) | 7.8 | 0.246 |
| Baseline eGFR | 1.43 | 0.04 |
| Baseline albumin / creatinine ratio in urine (mg / g) | 1.25 | 0.09 |
| Baseline potassium | 16.2 | 0.12 |
| Treatment group | 7.8 | 0.01 |
eGFR: estimated glomerular filtration rate; ABPM: ambulatory blood pressure monitoring; SBP: systolic blood pressure; DBP: diastolic blood pressure.
Spironolactone treatment reduced albuminuria from 210 (121–385) mg / g to 65 (45–120) mg / g at the end of the follow-up (p<0.01). In contrast, no changes in albuminuria were observed in the furosemide group during the follow-up period, actually there was a non-significant rise in albumin/ creatinine index from 369 (249–690) mg / g to 615 (290–989) mg / g at the end the study (p=0.24). At baseline, patients receiving spironolactone had a lower albumin / creatinine ratio than those in the furosemide group, 210 (121–385) mg / g vs. 369 (249−690mg / g), although the difference was not statistically significant (p=0.09). A general linear model was performed to compare the percentage of variation of the albumin / creatinine ratio in the 2 treatment groups adjusted for the baseline values of SBP, DBP, eGFR, albumin / creatinine ratio and potassium levels. In the model, the only factor that was significantly associated with the percentage variation of the albumin / creatinine index was the treatment group (p=0.01) (Table 3).
General linear model for factors associated with the percente variation in the albumin / creatinine ratio.
| F | p | |
|---|---|---|
| Mean baseline SBP (24h ABPM) | 41.8 | 0.12 |
| Mean baseline DBP (24h ABPM) | 10.6 | 0.09 |
| Baseline eGFR | 9.21 | 0.06 |
| Baseline albumin / creatinine ratio in urine (mg / g) | 6.21 | 0.11 |
| Baseline potassium | 13.2 | 0.31 |
| Treatment group | 6.8 | 0.01 |
eGFR: estimated glomerular filtration rate; ABPM: ambulatory blood pressure monitoring; SBP: systolic blood pressure; DBP: diastolic blood pressure.
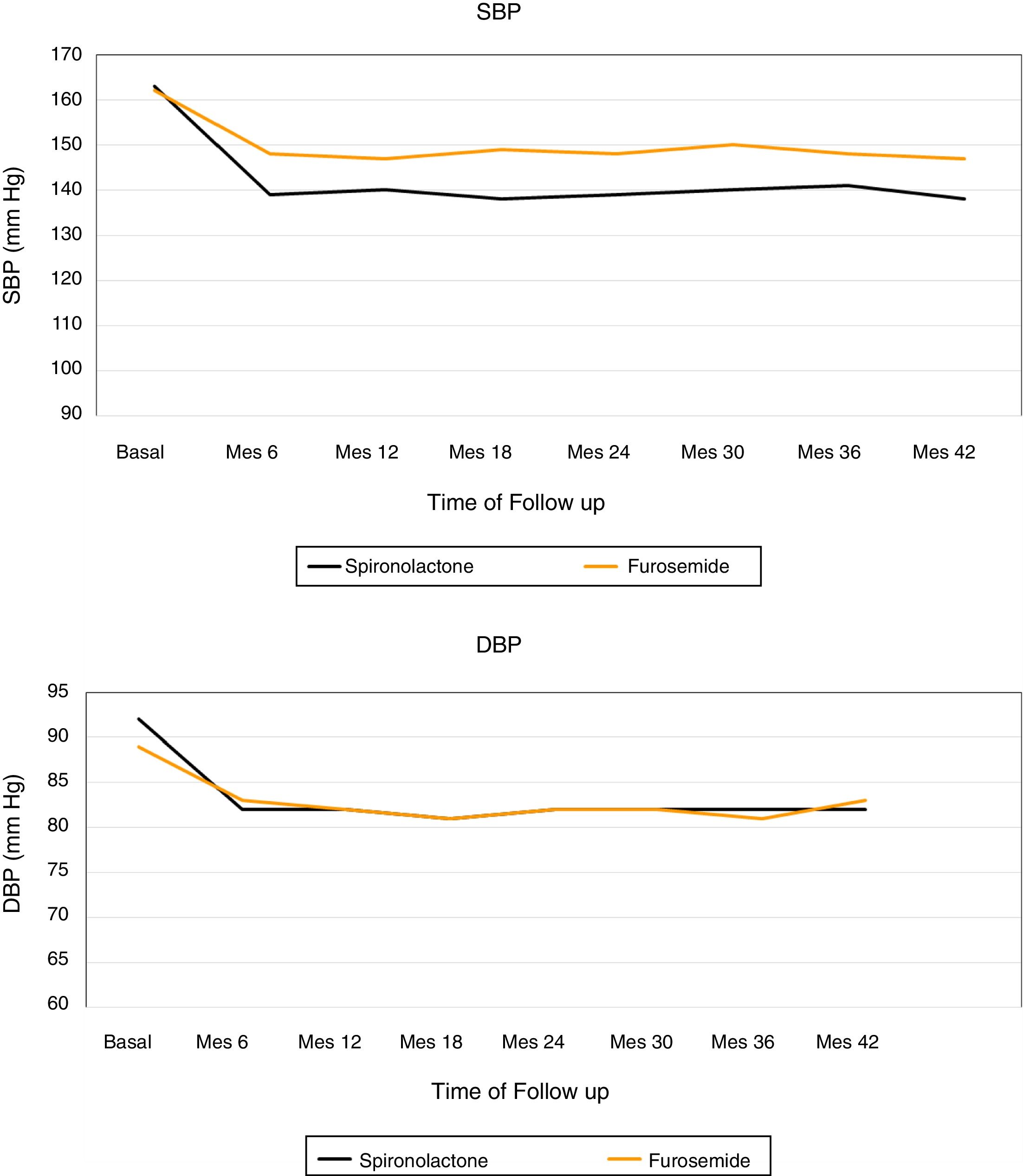
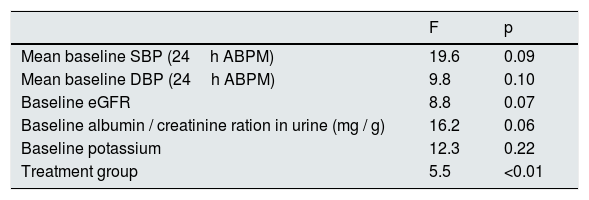
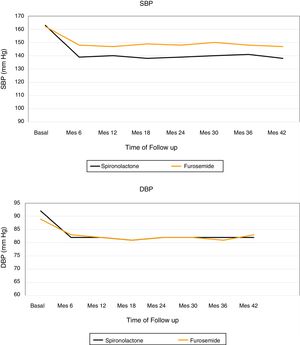
Fig. 2 shows the evolution of the mean SBP and DBP from the 24h measurement ABPM in the 2 treatment groups. At the end of the follow-up, in patients treated with spironolactone, the SBP decreased by 23±9mmHg (from 163±8 to 140±8mmHg), as compared with a decrease of 16±3mmHg (from 162±8 to 146±7mmHg) in the furosemide group. A general linear model was perfomed to compare the decrease in SBP in the 2 adjusted treatment groups for the baseline values of SBP, DBP, eGFR, albumin / creatinine index and potassium levels. The decrease in SBP was significantly associated only with the treatment received (p<0.01) in the adjusted model (Table 4).
General linear model for factors associated with the decrease in SBP.
| F | p | |
|---|---|---|
| Mean baseline SBP (24h ABPM) | 19.6 | 0.09 |
| Mean baseline DBP (24h ABPM) | 9.8 | 0.10 |
| Baseline eGFR | 8.8 | 0.07 |
| Baseline albumin / creatinine ration in urine (mg / g) | 16.2 | 0.06 |
| Baseline potassium | 12.3 | 0.22 |
| Treatment group | 5.5 | <0.01 |
eGFR: estimated glomerular filtration rate; ABPM: ambulatory blood pressure monitoring; SBP: systolic blood pressure; DBP: diastolic blood pressure.
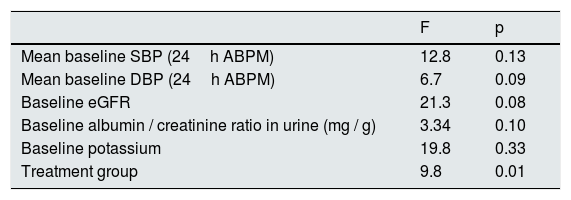
The DBP was reduced by 10±8mmHg in the spironolactone group and 6±2mmHg in the furosemide group. Again, a general linear model was performed to compare the decrease in DBP in the 2 adjusted treatment groups for the baseline values of SBP, DBP, eGFR, albumin / creatinine ratio and potassium levels, and it was observed that the only factor associated with the decrease in DBP was the treatment received (p=0.01) (Table 5).
General linear model for factors associated with the decrease in DBP.
| F | p | |
|---|---|---|
| Mean baseline SBP (24h ABPM) | 12.8 | 0.13 |
| Mean baseline DBP (24h ABPM) | 6.7 | 0.09 |
| Baseline eGFR | 21.3 | 0.08 |
| Baseline albumin / creatinine ratio in urine (mg / g) | 3.34 | 0.10 |
| Baseline potassium | 19.8 | 0.33 |
| Treatment group | 9.8 | 0.01 |
eGFR: estimated glomerular filtration rate; ABPM: ambulatory blood pressure monitoring; SBP: systolic blood pressure; DBP: diastolic blood pressure.
During the follow-up, 10 patients (33.3%) reached the target of a 24h mean BP byABPM < 130/80mmHg (there were 7 patients [46.6%] in the spironolactone group and 3 patients [20%] in the furosemide group; p<0.01). During the follow-up, in the furosemide group the number of antihypertensive drugs had to be increased from 3.7±0.5 to 5.1±0.9 drugs / day (p<0.01), while in the of Spironolactone group the increase in the number of antihypertensive drugs was not significant, from 3.9±1 to 4.3±0.5 drugs / day.
At baseline, 60% of the patients presented a non-dipper pattern on the ABPM, 20% had a riser pattern and 20% had a dipper pattern. Six months after treatment adjustment, it was observed a decrease in patients with a non-dipper and riser pattern to 53.3% and 13.3% respectively, and instead patients with a dipper pattern increased to 33.3%. We found no significant differences in the ABPM patterns in the 2 treatment groups.
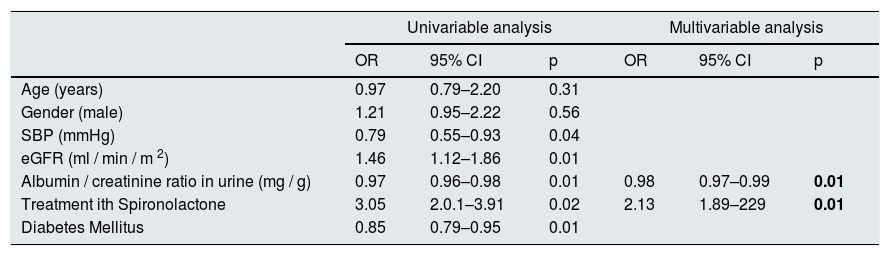
The average decrease in eGFR in the all patients was –2.8±5.4ml / min / 1.73m2. There were 14 patients with a rapid decrease in eGFR (greater than average) and 16 patients with a slow decrease in eGFR (less than average). Patients with a slow decrease in eGFR had lower values of SBP at baseline (159±8 vs. 168±10mmHg, p=0.04), a higher eGFR (63±16 vs. 45±14ml / min / m, p=0.01)), and less degree albuminuria, 160 (111–305) mg / g vs. 499 (243–954) mg / g, p=0.01, less percent of DM (37.5 vs. 78.5%, p=0.01) and most of them belong to the group that received spironolactone (64.2 vs. 37, 5%, p=0.02).
We performed a logistic regression model to identify factors associated with the slower decrease in eGFR, adjusted to sex, age, eGFR, DM and BP. The factors that were independently associated with a slower progression of CKD were: less albuminuria and treatment with spironolactone (Table 6).
Univariable and multivariable statistical analysis of factors associated with a slow progression of CKD.
| Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| Age (years) | 0.97 | 0.79–2.20 | 0.31 | |||
| Gender (male) | 1.21 | 0.95–2.22 | 0.56 | |||
| SBP (mmHg) | 0.79 | 0.55–0.93 | 0.04 | |||
| eGFR (ml / min / m 2) | 1.46 | 1.12–1.86 | 0.01 | |||
| Albumin / creatinine ratio in urine (mg / g) | 0.97 | 0.96–0.98 | 0.01 | 0.98 | 0.97–0.99 | 0.01 |
| Treatment ith Spironolactone | 3.05 | 2.0.1–3.91 | 0.02 | 2.13 | 1.89–229 | 0.01 |
| Diabetes Mellitus | 0.85 | 0.79–0.95 | 0.01 | |||
IC: confidence interval; OR: odds ratio; SBP: mean 24h systolic blood pressure by ABPM.
During the follow-up there were a total of 3 cardiovascular events: 2 cases of congestive heart failure (one in the spironolactone group and one in the furosemide group) and one amputation of the lower limbs in a patient with DM in the furosemide group.
No serious adverse events related to treatment adjustment were detected in any of the groups. Only one patient presented mild gynecomastia in spironolactone group, but did not require the suspension of treatment. None of the patients were excluded from the study due to severe hyperkalemia or acute renal failure. Two patients developed mild hyperkalemia (serum potassium 5.0–5.5 mEq / l) and were treated with calcium polystyrene sulfonate. The serum potassium level increased on an average 0.6 mEq / l (from 3.9±0.2 to 4.5±0.3 mEq / l) in the group of patients receiving spironolactone; and in the group that received furosemide there was a non-significant decrease in potassium levels (from 4.4±0.4 to 4.1±0.4 mEq / l). In the furosemide group it was observed, an asymptomatic increase in serum uric acid levels of from 6.9±0.9 to 7.6±0.7mg / dl (p<0.01), that was not observed in the spironolactone group, from 7.4±1.1 to 7.3±0.9mg / dl (p=0.41). Five patients (33%) had a uric acid level >8mg / dl and had to be started on allopurinol 100mg.
DiscussionAccording to the results obtained in the present study, in patients with resistant HTN long term treatment with spironolactone is more effective than a loop diuretic, furosemide, for the control of BP and proteinuria, and it was associated with a slower progression of CKD.
Despite the close relationship between resistant HTN and CKD, there is limited published data about the impairment of renal function in patients with resistant HTA and there is even less data on the effect of strategies aiming to control the BP on the progression of CKD of these patients.3,11 Our study shows a first piece of information on the long-term (3 years) progression of CKD in patients with resistant hypertension treated by 2 optimization guidelines for antihypertensive treatment (diuretic intensification with loop diuretics or with aldosterone blocker).
The few studies that have analyzed the progression of CKD in patients with resistant hypertension have found an important association between resistant hypertension and a rapid fall in eGFR. In the study by Kaboré et al.12 in 4265 patients with HTN and > 65 years, a 6.5% of the patients had resistant HTN. During a 4-year follow-up, the average drop in eGFR was twice as fast in the group of patients that met criteria for resistant HTN than in non-resistant HTN (–3.4±4.1ml / min / 1.73m2 per year versus 1.5±2.9ml / min / 1.73m2 per year, p>0.01); this decrease in eGFR was faster than in our study, in which the average drop in eGFR was –2.8±5.4ml / min / 1.73m2 per year. This difference may be related to the use of spironolactone in half of our patients in which the reduction of eGFR was with a slower than with furosemide of –2.1±4.8 versus –3.2±5.6ml / min / 1 73m2. This reduction in the progression of CKD is probably due to the better control of BP and proteinuria patients on spironolactone. Both higher BP and proteinuria are factors that have been classically associated with a fast progression of CKD; an improved control of these variables using various strategies is associated with a reduction in progression of CKD.13–15
Other studies have described that the treatment with aldosterone receptor antagonists produces a well control of BP and albuminuria.16 In the study conducted by Bianchi et al.17 in hypertensive patients with CKD, treatment with low doses of spironolactone (25mg / day) had a decrease in BP and albuminuria, that was associated with reduction in the fall of eGFR. The reduction in eGFR in the group receiving spironolactone for one year was similar to that observed in our group treated with aldosterone blockers : –0.323±0.04ml / min / 1.73m2 per month as compared to −0.474±0.03ml / min / 1.73m2 in the rest of patients (p<0.01). This nephroprotective effect of aldosterone antagonists has been attributed to the control of BP and albuminuria and also to the blockade of aldosterone effect itself. Recent experimental studies have shown that excess of aldosterone, as observed in many patients with resistant HTN, has detrimental effects in glomeruli and tubulointerstitial tissue. The increase in aldosterone produces podocyte damage, glomerurosclerosis, tubulointerstitial inflammation and fibrosis, increased oxidative stress and endothelial damage. All these factors are associated with renal damage and progression of CKD. This injury could be reduced by the action of mineralocorticoid receptor antagonists.18,19
As observed in other studies, both intensification diuretic therapy and the increase in treatment with renin-angiotensin-aldosterone blockers were associated with a worsening of renal function during the first weeks of treatment, with a subsequent partially recovery.20,21 We observed that in the group that received spironolactone the eGFR decreased during the first month (–5.8ml / min / 1.73m2) and in furosemide group the eGFR was also reduced (–4.1ml / min / 1.73m2). In the study by Bianchi et al.,17 in patients with CKD, the addition of spironolactone to the antihypertensive treatment, produced an effect similar to the observed in our study, an initial decrease in eGFR of –5.1ml / min / 1.73m 2 (from 62.4±2, 4 to 57.3±2.7ml / min / 1.73m2). This effect has been related to a hemodynamic changes secondary the decrease in BP itself, a decrease in intraglomerular pressure, and the associated volume depletion caused by the diuretic. Thereafter, as seen in our patients, there was a partial recovery of the eGFR.
In contrast with other studies, we did not find a significant reduction of albuminuria with the use of furosemide. In nephropathies with proteinuria such as the diabetic nephropathy, the combination of loop diuretics and thiazides has been shown to reduce proteinuria.22 In the work by Morales et al.,23 in patients with proteinuria due to diabetic nephropathy on treatment with renin angiotensin system blockers, 3 different diuretic regimens were added: spironolactone, hydrochlorothiazide or amiloride+hydrochlorothiazide. Proteinuria was significantly decreased in the 3 groups but the reduction was more marked in the group treated with thiazides alone or with amiloride. The authors attributed the decrease in proteinuria to the decrease in BP and the reduction in intraglomerular pressure caused by volume depletion secondary to the use of diuretics, however they do not find a clear physiological explanation for the differences observed between the different types of diuretics since these effects are common to the 3 diuretic.
In our study, the mean values of 24h SBP measured with ABPM were significantly lower in the group of patients treated with spironolactone than in those treated with furosemide. This is despite the fact that in the furosemide group the number of antihypertensives drugs were increased during the follow-up from 3.7±0.5 to 5.1±0.9 (p<0.01), while in the spironolactone group there was not a significant increase in the number of antihypertensive drugs, from 3.9±1 to 4.3±0.5 / day. In previous studies, such as PATHWAY-2,24 that compared the effect of spironolactone with other antihypertensives, such as bisoprolol or doxazosin, aldosterone antagonists, it appeared that spironolactone is more effective than the rest of the other medications in the control of BP control in patients with uncontrolled resistant hypertension.
Both furosemide and spironolactone were safe and reasonably well tolerated in our study. In the spironolactone group, it was observed an increase in serum potassium levels, however toxic hyperkalemia was not observed, this may have to do with the frequent analytical controls and the dietary teaching to patients in the Nephrology outpatient clinics. Patients with CKD, especially diabetics, have a higher risk of hyperkalemia than the general population due to the high prevalence of type IV tubular acidosis; therefore it is required a close control of serum potassium levels. In the work of van Buren et al.25 a greater increase in serum potassium levels was reported in patients with diabetic nephropathy treated with spironolactone than with losartan, despite a similar urinary excretion of sodium and potassium. This findings highlights the importance of extrarenal homeostasis of potassium in this population. Previous studies have shown that the risk of hyperkalemia is high in elderly patients, with DM, CKD and high basal potassium levels; these patients require a close monitoring of serum potassium levels. In our cohort, one of the patients (6.7%) treated with spironolactone developed mild gynecomastia, although it was not necessary to discontinue the medication. Similar rates of gynecomastia, around 10%, have been described in other studies.26 An asymptomatic increase in uric acid levels was observed in the furosemide group, a very common side effect seen with the use of this type of diuretic.27
The present study has some limitations, it is observational, prospective but non-randomized, however our study is the only one that evaluates the progression of CKD in patients with resistant hypertension treated by 2 antihypertensive therapies (furosemide or spironolactone added to a previous antihypertensive regime). A limitation in the interpretation of the results of this observational study with a limited population of 30 patients is that, although it was not statistically significant, there were some baseline differences between the 2 groups of patients. The eGFR and in the albumin / creatinine ratio in urine, were more favorable in the group that received spironolactone. Again these differences were not statistically significant. Generalized linear models adjusted to baseline characteristics indicate that the most relevant factor associated with the fall of eGFR, the control of BP and the decrease in albuminuria was the treatment received: spironolactone vs furosemide; regardless of the baseline characteristics of the patients. Another limitation of the study is the use of 2 drugs, spironolactone and furosemide, with different pharmacokinetics, so the sub optimal control of BP with furosemide could be related to insufficient dosing. Nevertheless, in a previous study of our group,7 in which we used bioimpedance to adjust the dose of furosemide in patients with resistant HTN, it was observed that furosemide administered once a day is very useful reducing the extracellular volume and the values of BP.
Our findings, although modest, contributes to the current knowledge on the optimal strategy for BP control in patients with resistant HTN. Spironolactone, with its potential positive effect on the reduction of progression of kidney disease, should be explored in subsequent randomized studies with a larger number of patients, that allow the examine its possible beneficial effect on cardiovascular events and mortality.
FinancingNo funding has been received to perform the present study.
Conflict of interestsNone of the authors have a conflict of interest.
Please cite this article as: Verdalles U, Goicoechea M, García de Vinuesa S, Torres E, Hernández A, Verde E, et al. Progresión de la enfermedad renal crónica en pacientes con hipertensión resistente sometidos a 2 estrategias terapéuticas: intensificación con diuréticos de asa vs. antagonistas de la aldosterona. Nefrologia. 2020;40:66–74.