Chronic interstitial nephritis in agricultural communities (CINAC) is a chronic tubulointerstitial nephropathy with extrarenal manifestations that cannot be attributed solely to CKD. CINAC typically affect young to middle age male farmworkers but and also women and children living in the same areas.1 A recent publication suggested that the pathologic abnormalities seen in CINAC may represent a toxin-induced nephropathy caused by factors that affect calcineurin signaling. Herbicides and insecticides utilized in areas with high rates of CINAC, have documented calcineurin inhibition effects leading to a strikingly similar proximal tubular lesion in this form of nephropathy.2
In 2017, El Salvador reported elevated rates of obstetric morbidity (8%), mainly due to pre-eclampsia. One hospital, which serves the eastern region of the country where CINAC is more prevalent, reported much higher rates of obstetric morbidity, 33.8% of births from urban areas and 64.1% of births from rural areas.3
We report the case of a 27-year-old healthy woman G1P1A0 with prenatal care for 3 weeks, who presented in 2016 to the national women's hospital at 32-week gestation with a diagnosis of pre-eclampsia. She had no family history of pre-eclampsia or kidney disease. Her past medical history revealed a creatinine of 1.3mg/dL at age 15 during a community health screening. She was exposed to pesticides while performing farm work from age 9 to 14 years, and had additional ongoing contact with lands irrigated with pesticides, she consumed water from wells until 18 years of age.
During the hospitalization she was diagnosed with severe pre-eclampsia and underwent emergency C-section without complications. A baby girl was delivered without complications, birth weight 2500g, height 47cm, head circumference 33cm. The patient was discharged two days after delivery with a blood pressure of 110/72mmHg and a serum creatinine of 1.5mg/dL she was referred to nephrology for further evaluation (Table 1). She continued follow-up every 3 months for 3 years. In 2019, three years post-delivery, she had a persistently elevated creatinine at 1.4mg/dL. Kidney Ultrasound showed right kidney measurements 10.6cm×5.7cm×5.9cm with a cortical thickness of 1.4cm. The left kidney measured 11.1cm×4.2cm×4cm with a cortical thickness of 1.9cm. There was no echogenicity, cysts, calculi, or masses. Normal bladder.
Clinical characteristics at specified time points.
| Clinical and laboratory data | Pre-eclampsiaAdmission 3/1/2016 | KidneyBiopsy9/3/2019 |
|---|---|---|
| Blood pressure (mmHg) | 146/100 | 110/80 |
| BMI (kg/m2) | – | 23.4 |
| Creatinine (0.6–1.3mg/dL) | 1.5 | 1.4 |
| GFR (mL/min×1.73m2 SC CKD-EPI) | 50 | 53 |
| Proteinuria (urinalysis) | Negative | Negative |
| Urea nitrogen (7–18mg/dL) | 13 | 12 |
| Uric acid (2.6–7.20mg/dL) | 11.4 | 5.2 |
| Glucose (70–115mg/dL) | 75 | 82 |
| Total cholesterol (<200mg/dL) | 168 | 173 |
| Triglycerides (35–150mg/dL) | 110 | 108 |
| Hemoglobin (11.50–18g/L) | 11.9 | 11.2 |
| Platelets (150–450 109L–1) | 352 | 273 |
| Na+ (135–145mmol/L) | 136 | 138 |
| K+ (3.5–5.5mmol/L) | 3.6 | 3.7 |
| Cl− (101–111mmol/L) | 101 | 107 |
| Mg++ (1.7–2.2mg/dL) | 6.8 | 1.8 |
| Ca++ (8.4–10.2mg/dL) | 9.5 | 8.8 |
| 24-h urine volume (mL) | 1800 | 1700 |
| Proteinuria in 24h (<0.015g) | 0.4 | 0.3 |
| Total protein (6.4–8.3g/dL) | 6.1 | – |
| Albumin (3.4–4.8g/dL) | 2.7 | 4.4 |
| ALT (10–40UI/L) | 10 | 12 |
| AST (10–42UI/L) | 22 | 14 |
| LDH (135–225 UI/L) | 146 | 136 |
| Total bilirubin (0.2–1.2mg/dL) | 0.4 | – |
| Direct bilirubin (0.0–0.5mg/dL) | 0.1 | – |
| Indirect bilirubin (0.2–1.35mg/dL) | 0.3 | – |
| HIV status | Negative | Negative |
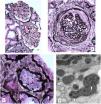
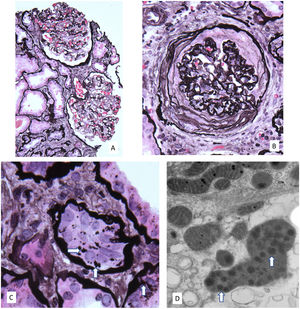
A kidney biopsy was performed a sample of kidney cortex contained 29 glomeruli, 11 of which were globally sclerotic (Fig. 1). Glomeruli were mildly enlarged and four had severe ischemic-type capillary wall corrugation with segmental deposition of collagen in the urinary spaces. There were no segments of sclerosis, glomerular inflammation, mesangial hypercellularity or crescents. There was 25% tubular atrophy with interstitial fibrosis and a mild associated interstitial lymphocytic infiltrate without neutrophils or eosinophils. Focal atrophic and preserved proximal tubules had epithelial cells with large irregular silver positive cytoplasmic granules. Arteries had mild muscular hypertrophy and mild intimal fibrosis, and arterioles were unremarkable. Immunofluorescence negative. By electron microscopy, glomeruli with wrinkled capillary walls had thickened and corrugated basement membranes with severe podocyte foot process effacement. The glomeruli showed normal basement membranes with 25% podocyte foot process effacement. The cytoplasm of focal proximal tubular cells had clusters of ovals to mildly dysmorphic small lysosomes containing peripheral electron dense aggregates as well as fewer large dysmorphic lysosomes containing dispersed electron dense aggregates. Features consistent with CINAC with mild arterial nephrosclerosis.
(A) Moderately enlarged glomeruli without segmental lesions or hypercellularity. There is focal tubular atrophy with interstitial fibrosis without inflammation (periodic-acid methenamine silver, original 80×). (B) Global ischemic-type capillary wall corrugation and retraction with collagen accumulating in the urinary space. Note the normal arteriole in the upper left (periodic-acid methenamine silver, original 160×). (C) Atrophic proximal tubular cells containing enlarged irregularly shaped silver positive granules (arrows) (periodic-acid methenamine silver, original 240×). (D) Electron microscopy showing an enlarged dysmorphic lysosome containing dispersed electron dense aggregates (arrows) in a medium electron dense matrix within a proximal tubular cell (original 20,000×).
CKD is a risk factor for pre-eclampsia but additional risk for pregnancy complications may be seen with specific renal disorders.5 Pesticide exposure has been associated with hypertensive disorders during pregnancy in the US.6 Organochlorine pesticides have been identified in the umbilical cord blood of exposed mothers living in Mexico, with higher concentrations associated with longer exposure to environmental contaminants.4 Toichuev et al. identified a highly significant increase in the relative risk of neonatal and maternal health problems, including pre-eclampsia, with increasing levels of placental organochlorine pesticides in a concentration-dependent manner.7
Suggested mechanisms for pesticides causing pre-eclampsia include oxidative stress, endocrine disruption, abnormal placental vascularization and epigenetic changes.8 In contrast, a recent study by Karunananda did not identify placental abnormalities in postpartum women living in endemic areas in Sri Lanka; however, it is unknown if this is true for women who have CINAC renal lesions with associated impaired eGFR versus those women who just reside in an endemic region.9
Other than CKD, this patient had no typical risk factors for pre-eclampsia, such as hypertension, diabetes, obesity, smoking, a family history of pre-eclampsia, advanced maternal age, or multiple pregnancies.10 She had life-long exposure to agrochemicals and kidney biopsy consistent with CINAC, suggesting related end organ damage. These findings suggest a link between these exposures and hypertensive disorders of pregnancy and highlight the need for more extensive and prospective studies.
Conflicts of interestNone.