Proteinuria is common following kidney transplantation and affects more than 40% of kidney transplant patients per year. In general, the level of proteinuria is low (<500mg/day) but even those levels significantly reduce graft and patient survival. This is why it is of vital importance to detect proteinuria quickly following transplantation and to investigate its cause. During the same year of the transplant, proteinuria may be caused by multiple factors, including glomerular disease, effects of anti-HLA class II antibodies and drugs such as mTOR inhibitors, tubulointerstitial disease of the graft, and significant functional discrepancy between the graft and the recipient. The relationship between proteinuria and graft survival is likely to be due to the factors that cause proteinuria. It is unknown why proteinuria and patient survival are related, but it could be due to a relationship between proteinuria and traditional cardiovascular risk factors or a relationship between proteinuria, endothelial function and inflammation. To treat proteinuria, three aspects should be considered: the cause of proteinuria, the non-specific reduction of proteinuria, and the reduction of the cardiovascular risk.
La proteinuria es frecuente después del trasplante renal y afecta a más del 40% de los pacientes al año del mismo. En general, el nivel de proteinuria es bajo (<500 mg/día), pero incluso estos niveles se relacionan con una reducción significativa en la supervivencia del injerto y del paciente. Por este motivo es importante detectar la proteinuria rápidamente después del trasplante e investigar su causa. Al año del trasplante, la proteinuria se puede deber a múltiples causas, entre las que se incluyen enfermedad glomerular, efecto de anticuerpos anti-HLA de clase II, efecto de fármacos como los inhibidores de m-TOR, enfermedad túbulo-intersticial del injerto y discrepancia funcional importante entre el injerto y el receptor. La relación entre la proteinuria y la supervivencia del injerto probablemente sea la causa de la proteinuria. Los motivos de la relación entre la proteinuria y la supervivencia del paciente se desconocen, pero pueden deberse a una relación entre proteinuria y factores de riesgo cardiovascular tradicionales o a una relación entre la proteinuria, la función endotelial y la inflamación. El tratamiento de la proteinuria debe considerar tres aspectos: la causa de la proteinuria, la reducción inespecífica de la proteinuria, y la reducción del riesgo cardiovascular.
INTRODUCTION
Proteinuria is common after kidney transplantation and affects between 35%-45% of patients during the same year as their transplant.1 Traditionally, the literature on post-transplant proteinuria used to discuss the differential diagnosis of high-grade proteinuria and its relationship with the patient’s graft survival.2 Over the last five years, we have learnt that proteinuria, at all levels, is an important biological marker used to identify patients and grafts with a poor prognosis. These studies suggest two questions that we shall try to answer in this review: What causes low- and high-grade proteinuria after transplantation? and, why is proteinuria associated with reduced patient and graft survival?
PREVALENCE
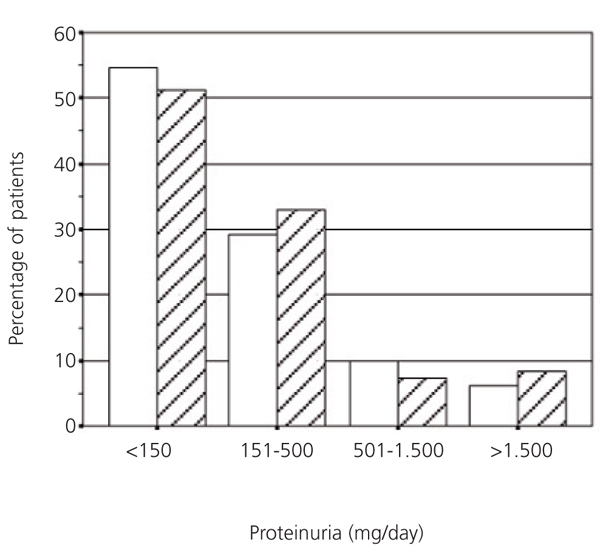
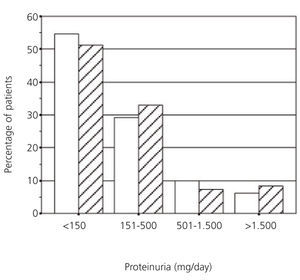
In different studies, the prevalence of proteinuria varies between 15% and 45%. This variation is mainly due to differences in the level of proteinuria used to define the value considered as abnormal,1 and when proteinuria was determined. We believe that it is important to diagnose proteinuria during the first few months after the transplant, which allows us to identify the patients and grafts that are at high risk. Figure 1 shows the prevalence of proteinuria a year after transplantation in living- and cadaver-donor kidney transplant recipients. As can be observed, there are no significant differences in either prevalence or proteinuria levels among these two groups. Figure 1 also shows that post-transplant proteinuria is generally low-grade, and therefore, 30% of patients have between 150mg/day and 500mg/day, although 6.5% of recipients in this group have proteinuria greater than 1500mg/day.
Data on the prevalence of albuminuria after transplantation is scarcer. In studies conducted in our department, we found that albuminuria is common, and that it affects most patients with proteinuria, even when levels are low. For example, more than 80% of patients with proteinuria between 150mg/day and 500mg/day, and 100% of patients with higher proteinuria levels have albuminuria.3 Of the patients that do not have proteinuria, approximately 15% have albuminuria levels above 30mg/day.3
PROTEINURIA CAUSES
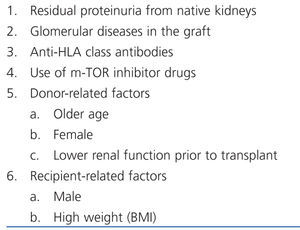
Post-transplant proteinuria could be due to several causes (Table 1). Patients with high-grade proteinuria (>1500mg/day) frequently have graft glomerular diseases (80%).3 However, it may be difficult to determine what caused proteinuria in patients that have lower levels. Below, we briefly explain the different causes of proteinuria.
Residual proteinuria
The presence of proteinuria from native kidneys may make it harder to interpret proteinuria detected after transplantation. This is common in patients that receive a kidney transplant before or slightly before starting dialysis, and therefore with a significant kidney function and residual diuresis.4 Results from two studies serve as practical guidelines to help us interpret proteinuria in these patients. Firstly, these studies show that pre-transplant proteinuria, even within the nephrotic range, abruptly reduces during the first weeks after receiving a normal functioning kidney transplant.4,5 This reduction is probably due to the reduction in the blood flow which occurs in native kidneys after a transplant, if the graft is functioning correctly.5 This last point is important because, from our experience, the blood flow in the native kidneys is maintained and the ‘native’ proteinuria persists for recipients with a poor functioning graft. A second study helps us interpret proteinuria.4 Firstly, for patients with a normal functioning graft, the presence of proteinuria above 3000mg/day three weeks after the transplant should not be attributed to native kidneys but indicate the presence of glomerular disease in the graft (probably recurrent glomerulopathy). Secondly, proteinuria above 1500mg/day a year after the transplant and/or an increase by more than 500mg/day in proteinuria from the third week to 1 year after the transplant indicates a disorder in the graft. Thirdly, native kidneys may have low levels of proteinuria (less than 500mg/day) even a year after the transplant, although it is expected that the proteinuria would decrease over time.
Glomerular diseases in the graft
In a previous study, we have assessed protocol biopsies in patients with proteinuria a year after transplant.3 Only 9% of these patients had a glomerular disease. However, in 80% of patients with proteinuria above 1500mg/day there was evidence of glomerular disease. Other studies support these results.2 We must consider three types of glomerular disease in the graft: recurrent diseases, de novo diseases, and post-transplant glomerular disease.
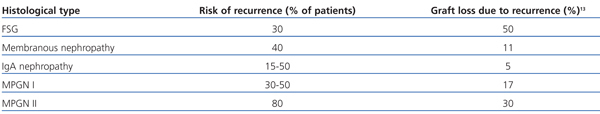
Table 2 summarises the prevalence and the consequences of most common recurrent glomerular diseases in the graft. It highlights that in most studies, recurrence has been diagnosed based on the presence of proteinuria. Focal segmental glomerulosclerosis (FSG) affects 30% of patients and is associated with a high risk of recurrence. Previous studies have identified subgroups of patients with FSG which have a much higher risk, including: patients diagnosed before being 18 years old, patients with rapid disease progression (less than three years)6 and, in particular, patients with a history of recurrences in previous transplants. Approximately 50% of patients with recurrent FSG lose their graft. Membranous nephropathy (MN) is also associated with a high risk of recurrence. Protocol biopsy studies showed that MN’s histological recurrence generally occurs in 40% of cases during the first few months after the transplant, and that histological changes do not initially cause proteinuria.7 IgA nephropathy frequently occurs (>50%) after the transplant, although histological changes are generally mild: IgA deposits detected by immunofluorescence and small mesangial deposits detected by electron microscopy. However, mesangial cells do not proliferate and there are no clinical signs. It is rare for recurrent IgA nephropathy to cause graft loss, although we have observed cases of aggressive clinical behaviour and high-grade proteinuria. Mesangiocapillary glomerulonephritis (or membranoproliferative glomerulonephritis, MPGN) has raised great interest lately given new data that allow us to differentiate between several subtypes of this disease.8,9 Considering recurrence, differentiating between these types of MPGN has important clinical consequences. For example, in cases of MPGN associated with monoclonal proteins, the disease could rapidly recur and have an aggressive clinical presentation.10 Patients with MPGN type 1 and hypocomplementaemia have a high risk of reccurrence.10,11 In general, for patients with MPGN type 1 or 2 and hypocomplementaemia, complement alterations should be suspected9,12 and if they do exist, prognosis following the transplant is severe.
Protocol biopsy studies revealed that glomerular disease recurrence is not associated with proteinuria in many cases.7,10 This is interesting for many reasons: firstly, histological diagnosis by protocol biopsies provides us with information about when the recurrence occurs and on the histological changes that are produced during the initial phases of these diseases for the first time. Secondly, it is reasonable to believe that treating these recurrences in early stages is more effective than in more advanced stages. Thirdly, we must remember that low-grade proteinuria can be the first sign of a recurrent native kidney glomerular disease, which could have negative consequences for the graft in the long-term. Therefore, it is important to examine those patients with low-grade proteinuria and closely monitor them, using regular measurements and performing a biopsy if proteinuria gradually increases.
In a number of cases, the graft develops a glomerular disease that is not recurrent, but which does have the histological characteristics of a native kidney glomerular disease. These glomerular diseases are generally diagnosed later than recurrent ones and could cause the graft to be lost.13 The most common histological types include FSG,14 MN, and mesangiocapillary glomerulonephritis. These diseases are not very common and their aetiologies are poorly defined.
Post-transplant glomerular disease (PG) has been recognised for many years as a disease that is generally diagnosed several years after transplantation and that could cause high-grade proteinuria, even nephrotic range proteinuria.15,16 In recent years, we have learnt that this disease, in most cases, is due to the damage produced to the capillaries by the anti-HLA class II antibodies.17 Using protocol biopsies and being able to measure these antibodies effectively has meant that we are able to know that PG develops during the first few months after the transplant, and that the clinical presentation frequently consists in gradual loss of graft function with severe hypertension and low-grade proteinuria. The proteinuria grade is an important prognostic index in these patients.13
In more recent studies (presented in the American Transplant Congress [ATC] 2011), we have shown that proteinuria is an early marker of damage produced to the glomerular capillaries by anti-HLA class II antibodies, before histological signs of PG are visible. When proteinuria is present in patients with anti-HLA class II antibodies, it identifies a subgroup of patients with a high incidence of glomerulitis and high risk of developing PG in the future. Electron microscopy studies showed that damage to glomerular capillary endothelial cells precedes the histological changes, which we consider to be diagnostic of PG.18
Tubular and interstitial damage and proteinuria
Studies published many years ago attributed proteinuria to “chronic rejection of kidney transplants”.15 In most cases, biopsy showed changes in PG. Biopsy studies in patients with proteinuria3 showed that there is a subgroup of patients with post-transplant chronic kidney disease (interstitial fibrosis and tubular atrophy19), free of glomerular disease, but with low levels of proteinuria. These patients often present with albuminuria3 and, therefore, it is possible that they have a hidden glomerular disease. Furthermore, the proximal tubule reabsorbs large quantities of albumin,20 meaning that proteinuria in these patients could be due to tubular damage. Although we do not know the origin of the proteinuria in these patients, we should mention that there are not many cases of graft survival.3
m-TOR inhibitor-induced proteinuria
The use of sirolimus or everolimus has been associated with post-transplant proteinuria in numerous studies.1,21,22 Initially, this observation occurs in patients with post-transplant chronic kidney disease after converting from calcineurin inhibitors (CNI) to sirolimus so as to preserve kidney function. For this reason, it was suggested that the sirolimus-associated proteinuria was the result of the CNI-induced haemodynamic effect. At this time, evidence indicates that m-TOR inhibitors directly affects protein filtration in the glomerulus and specifically in the podocyte. Letavernier et al23 showed that sirolimus affects the VEGF, which is essential for podocyte and endothelial cell survival, and for the intercellular Akt signalling, which is critical for epithelial cell differentiation, adhesion and survival. The most recent studies have shown a reduction in protein expression that constitute the slit-diaphragm, an important structure for the glomerulus’ selective ultrafiltration.24,25
Clinical studies indicate that the presence of proteinuria in a sirolimus-treated patient can have negative consequences on the graft. CNI conversion to sirolimus was found to have negative effects on graft survival, especially in patients with proteinuria.26 In isolated cases27 FSG development has been detected in sirolimus-treated grafts. m-TOR inhibitor drugs can cause protein to increase significantly and kidney function to deteriorate, especially in patients with incipient glomerular diseases (mainly recurrent IgA). In contrast, there is no clear evidence that sirolimus is harmful to the graft in patients that maintain a low and stable proteinuria level (<500mg/day). Nevertheless, these patients should be monitored closely, measuring their proteinuria levels regularly, and if it is increases progressively, m-TOR inhibitor withdrawal should be considered.
Proteinuria related with donor and recipient factors
It is interesting that of all the patients with proteinuria a year after transplant, 51% have a clear cause. These include glomerular disease in the biopsy, use of sirolimus or anti-HLA class II antibodies. However, 49% of patients with proteinuria do not present with any of these factors. This observation made us examine other factors that could be related to proteinuria (Table 3) (abstract presented at the ATC, 2011).1
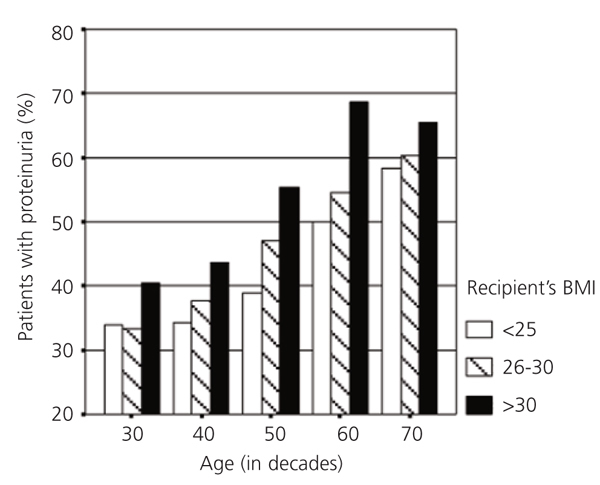
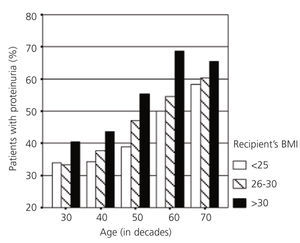
It is interesting to consider the relationship between proteinuria and the donor’s and recipient’s demographic factors.3 Proteinuria is more common and abundant in transplants from smaller donors or donors with less function (older donors, female donors, donors with relatively lower function) and in transplants for larger recipients (male recipients with a higher body mass index [BMI]). These data suggest that the difference in size/function between the donor and the recipient partly conditions the risk of proteinuria. Physiologically, it is possible that this size difference causes glomerular hyperfiltration, which could produce proteinuria and progressive kidney deterioration.28,29 One possible example of this model is shown in Figure 2, which highlights that the percentage of patients with proteinuria increases with the donor’s age. At the same time, in each donor group defined by age, the risk of proteinuria gradually increases as the recipient’s weight increases.
RELATIONSHIP BETWEEN PROTEINURIA AND GRAFT SURVIVAL
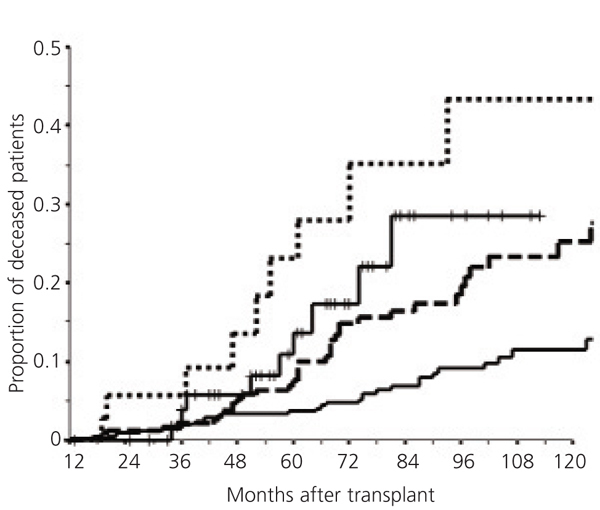
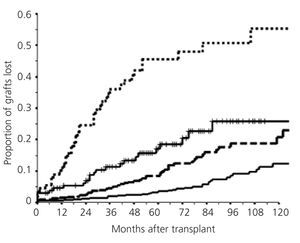
Interest in post-transplant proteinuria is basically due to its relationship with graft survival. In general, when the proteinuria level increases, graft survival decreases (Figure 3). This figure highlights that the risk of graft loss gradually increases with the level of proteinuria and that said risk is notable even for patients who we consider have a low level of proteinuria (<500mg/day). In previous studies,3 compared to proteinuria-free grafts, we calculated that the risk of graft loss with proteinuria levels between 150mg/day and 500mg/day increases 2.45 times, 6.07 times in patients with proteinuria between 501mg/day and 1500mg/day, and 14.3 times with proteinuria above 1500mg/day.
Why is there a relationship between the proteinuria level and graft survival? We suggest that proteinuria can be used as an indicator of a number of aggressions that may affect the graft, and that they, not proteinuria, are mainly the cause for graft loss. For example, it is very likely that the glomerulopathy is the main cause of the graft loss in patients with high levels of proteinuria due to glomerular disease. It is also possible that the presence of high levels of proteinuria may cause interstitial damage to the graft in these patients.30 It is a lot less clear why grafts with low levels of proteinuria also have a compromised survival,31,32 meaning that we should consider the cause of proteinuria once more (Table 1 and Table 3). Low levels of proteinuria can indicate: 1) the first stages of a glomerular disease recurrence; 2) the effect of anti-HLA class II inhibitors; or 3) an important disparity between the graft function and the recipient’s needs. In each of these cases, proteinuria is related to a reduction in graft survival, but the mechanism of graft injury is different. There are two types of low-grade proteinuria, which are not likely to be associated with an unfavourable prognosis: residual proteinuria and m-TOR inhibitor-induced proteinuria. Even in these cases, we have to be cautious and closely monitor the patient to confirm that it does not increase during the follow-up. If it does, we can rule out the possibility of proteinuria being residual4 and we must look for other causes.
To understand the relationship between proteinuria and graft survival, we must also consider that the presence of proteinuria is related to other donor, recipient and graft characteristics.3 For example, proteinuria is related to lower kidney function, and although it is important to consider this relationship, the relationship between proteinuria and graft survival is statistically independent of graft function.3
RELATIONSHIP BETWEEN PROTEINURIA AND PATIENT SURVIVAL
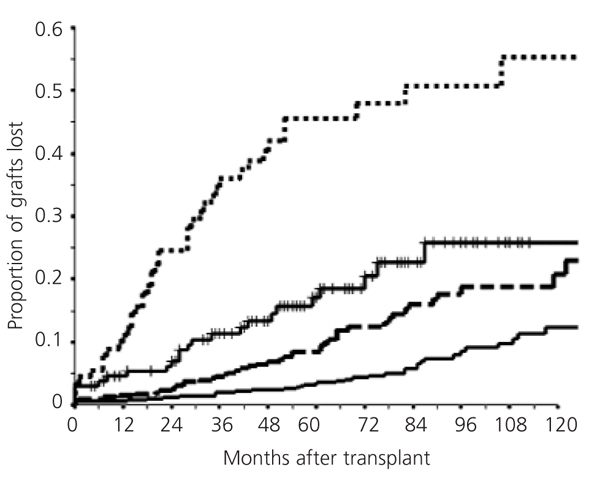
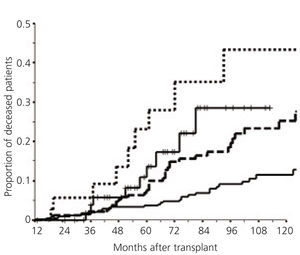
Post-transplant proteinuria is related to a reduction in patient survival. Several studies have shown this relationship.33-35 To be exact, other studies showed that cardiovascular risk increased by 2.45 times in patients with proteinuria, compared with patients without proteinuria.36 In more recent studies (presented in the ATC, 2011), our group confirmed the relationship between a gradual increase in proteinuria and a reduction in patient survival, and we started to study the factors that could explain this relationship (Figure 4). This figure also highlights that even low levels of proteinuria, less than 500mg/day, are related with a significant reduction in patient survival.
We must consider three types of factors to try and explain the relationship between proteinuria and patient survival: firstly, it is possible that the factors that cause proteinuria also increase the patient’s risk. Studying these factors (Table 3) does not suggest that this is a plausible explanation, in fact, preliminary studies have not found a relationship between the proteinuria-related factors and patient survival. Secondly, it is possible that patients with proteinuria acquire or have other factors that could be related with patient survival.3 We have recently extended these studies (ATC, 2011) and, proteinuria is indeed related with other biochemical parameters (increased lipids and reduced albumin and haemoglobin), blood pressure, and reduced graft function. All of these variables are related with patient survival. Thirdly, it is well known that, microalbuminuria is related with a higher mortality rate for all causes in the general population, including cardiovascular problems.37,38 This relationship is generally explained by the association between albuminuria/proteinuria and alterations in the endothelial function and/or inflammation. Recently, a retrospective study observed that microalbuminuria is related to kidney transplant survival.39 What was surprising from this study is that albuminuria was not only related to an increase in cardiovascular risk but also to an increase in the risk of cancer-related death. Post-transplant albuminuria is very common, therefore, it is important to confirm these relationships and study the factors that could explain the mechanisms underlying the relationship between proteinuria and kidney transplant survival.
TREATMENT
Three aspects should be considered when treating a patient with proteinuria: 1) specific treatment for the cause of proteinuria; 2) reduce proteinuria using non-specific treatments; and 3) cardiovascular risk treatment. Proteinuria treatment, especially for low-grade proteinuria, is often limited to controlling blood pressure or using angiotensin-converting enzyme inhibitors (ACEi), which can reduce the concentration of proteins in urine. Although this may probably be useful, in our opinion, it is wrong not to try and investigate the possible cause of proteinuria. Establishing the cause does not only give us the graft’s prognosis, it can also identify the mechanism of graft injury and it can open up the way for specific treatment.
Specific treatment
There is not enough scope in this review to be able to discuss in depth all of the therapies that we can use to treat multiple causes of post-transplant proteinuria (Table 1). In general, recurrent glomerular disease treatment follows the same regimens used to treat these diseases in the native kidney. However, when we treat these diseases in transplant patients we must consider several specific factors:
1. Using anti-rejection drugs may modify the behaviour of certain glomerular diseases in the graft. For example, if we consider that the prevalence of recurrence of these diseases is low, such as for lupus erythematosus. Furthermore, anti-rejection drugs may modify the effects of other drugs. For example, it is known that the anti-CD20 antibodies cause lymphocytes B to deplete over a much longer period in transplant patients than in other patients.40,41 From our experience, cyclophosphamide can be used to treat acute, aggressive glomerular diseases in the graft, but it should not be used at the same time as azathioprine or mycophenolate, given the high risk of leucopoenia.
2. Transplant patients obviously had a severe glomerular disease in the native kidney. Therefore, these diseases can be especially aggressive in transplant patients. This, of course, is not applicable to all patients, for example, the recurrence of IgA nephropathy is generally mild. However, other studies have suggested that the aggressiveness of the disease in the native kidney is reproduced in the graft, as is the case for FSG.42 We have also detected low spontaneous remission rates of MN in the graft: of the 34 patients diagnosed with recurrent MN using protocol biopsies and with minimum clinical signs, proteinuria gradually increased in 33 of them. We also observed that patients’ kidney disease histologically worsened in those cases for which follow-up biopsy was performed. These considerations have implications for determining when the patient with recurrence must be treated.
3. Glomerular diseases can be diagnosed in the graft in their initial stages, as proteinuria is measured regularly for these patients.43 In our department, protocol biopsies allow us to frequently diagnose these diseases when there are no clinical signs. It may be for this reason that certain treatments are more effective in the graft than in the native kidney. For example, plasmapheresis can be effective in treating certain cases of FSG44 and MPGN.10,45 Early diagnosis of these disorders could theoretically improve their response to treatment, as we have seen is the case of MN.40
It is useful to measure the level of anti-HLA antibodies in patients with proteinuria for several reasons: 1) most patients with post-transplant glomerular diseases have or have had anti-HLA class II antibodies, therefore, their presence has a diagnostic value; 2) the existence of anti-HLA class II antibodies and proteinuria is an early sign of capillary damage caused by antibodies and is associated with reduced graft survival; 3) the level of anti-HLA class II antibodies is related with graft survival and this relationship is independent of other factors such as markers in the biopsy (mainly the presence of C4d in the capillaries) and biochemical markers (proteinuria and kidney function);464) the development of new anti-HLA antibodies indicates that immunosuppression is not effective, either because the patient is not taking his or her medication regularly or because the indicated doses are not enough; 5) in patients that develop anti-HLA antibodies after the transplant, it is sometimes possible to suppress the level of antibodies with a higher dose of immunosuppressive agents and our recommendation is to increase the dose of mycophenolate and/or tacrolimus during a specific period of time (approximately six months), regularly measuring the serum antibody level.
In patients taking m-TOR inhibitors, regularly monitoring proteinuria is essential and if it has increased significantly we recommend withdrawing the drug. Sirolimus can spectacularly increase glomerular disease-induced proteinuria, even when it is mild. From our experience, withdrawing this medication improves proteinuria within a few months. Sirolimus can cause acute renal failure in patients with high levels of proteinuria.47 Finally, it is important to mention that converting from CNI to m-TOR inhibitors could be harmful for patients with proteinuria.26
As we have previously mentioned, we have discovered that approximately in 50% of patients post-transplant proteinuria may be related with donor or recipient factors indicative of an important disparity between the graft’s limited functional ability and the recipient’s needs. Possibly, in these cases, proteinuria is due to glomerular hypertension and glomerular hyper filtration and if this is the case, the inhibition of the renin-angiotensin system (RAS) must be considered as a specific treatment for the cause of proteinuria.48
Non-specific treatment of kidney transplant proteinuria
Based on results from studies on controlling proteinuria in chronic renal disease, it seems reasonable to recommend the following non-specific measures for patients undergoing transplant: 1) Control blood pressure (systolic blood pressure lower than 130mm Hg); 2) use ACEi and/or angiotensin II receptor blockers (ARB) at the highest tolerated dose, even if the patient does not have hypertension; 3) control the lipid profile, preferably using statins; 4) maintain an adequate BMI; 5) reduce the amount of proteins in the diet, and 6) stop smoking.49
Firstly, we must insist that these measures reduce the progression of kidney failure in non-transplant patients with renal problems, but, in general, they do not prevent its development.50 Therefore, we must consider these non-specific measures as treatment that should be used alongside specific treatment. Secondly, although RAS inhibition reduces post-transplant proteinuria, there is not enough evidence showing that it increases graft survival. Thirdly, it is important to highlight that using ACEi or ARB for transplant patients can have important negative consequences; therefore special precautions should be taken, i.e. using low doses and monitoring creatinine, electrolytes (potassium) and haemoglobin during the first weeks of treatment.
A systematic review51 of 21 randomised studies on the use of ACEi and/or ARB, which included 1549 transplant patients concluded that these drugs significantly reduced proteinuria, but they also reduced the levels of hematocrit and glomerular filtration. No significant differences were observed in blood pressure control nor in graft survival. However, studies included in this review have limitations given that they have a small sample size, reduced follow-up time and treatment was started at different times after the transplant. A prospective, randomised, controlled and multicentre study assessed the impact that an ARB (candesartan) had on graft survival and the cardiovascular morbidity and mortality in 502 kidney transplant patients.52 This study was finished before planned as there were less events than expected, but candesartan-treated patients’ proteinuria significantly decreased and they had a better blood pressure control than the patients who received a placebo. Two observational and retrospective studies tried to clarify the effect that RAS inhibition had on graft survival, but their results were contradictory. In the first,53 a significant positive effect was observed on graft and patient survival, regardless of the presence of proteinuria. In contrast, the second of these studies54 concluded that RAS inhibition does not have beneficial effects on graft survival or on patient survival, even in subgroups of patients with high cardiovascular risk. In summary, given the data that are currently available, it would be too early to recommend ACEi and/or ARB with the objective of improving graft or patient survival, as this is still unknown.
Cardioprotection
The presence of proteinuria, even at low levels, especially identifies patients with a reduced survival rate, but not only due to an increased cardiovascular risk.34,36 At present, the mechanism that causes this increased risk is still not known, and we do have enough data (as we have seen in the previous section) to conclude whether reducing proteinuria or the use of RAS inhibitors improves prognosis for these patients. However, it is reasonable to intensify the cardioprotective measures in patients with proteinuria. The following measures should be included: control blood pressure (lower than 130mm Hg); use statins maintaining an LDL-cholesterol level lower than 100mg/dl (less than 80mg/dl in high-risk patients55); tight control of glycaemia in diabetic patients (this measure is debatable in accordance with some recent studies56); stop smoking; use aspirin and use betablockers. The only measure that is proven, based on prospective, controlled and randomised studies, is that using statins is effective in transplant patients.57
KEY CONCEPTS
1. Post-transplant proteinuria is common and could be due to multiple causes.
2. Proteinuria, even low-grade proteinuria (<500mg/day), is a sensitive indicator of reduced graft survival.
3. The reduction in survival of a graft with proteinuria is probably due to the same problem that causes proteinuria. Therefore, the cause of post-transplant proteinuria must be investigated.
4. Proteinuria, even low-grade proteinuria (<500mg/day), is also associated with a reduction in patient survival mainly due to an increased cardiovascular risk.
5. To treat proteinuria, three aspects should be considered: the cause, its reduction with non-specific measures, and the reduction of associated cardiovascular risk.
Table 1. Causes of post-transplant proteinuria
Table 2. Risk of post-transplant recurrent glomerular diseases
Table 3. Factors related to an increase in the prevalence and intensity of proteinuria a year after transplantation
Figure 1. Prevalence of proteinuria in living-donor kidney recipients (white columns, n=1043) and cadaver-donor kidney recipients (striped columns, n=193) a year after the transplant
Figure 2. Prevalence of proteinuria a year after transplantation in living-donor kidney recipients according to recipient's age and weight
Figure 4. Relationship between proteinuria and transplant patient survival
Figure 3. Relationship between proteinuria and graft survival (excluding patient death)