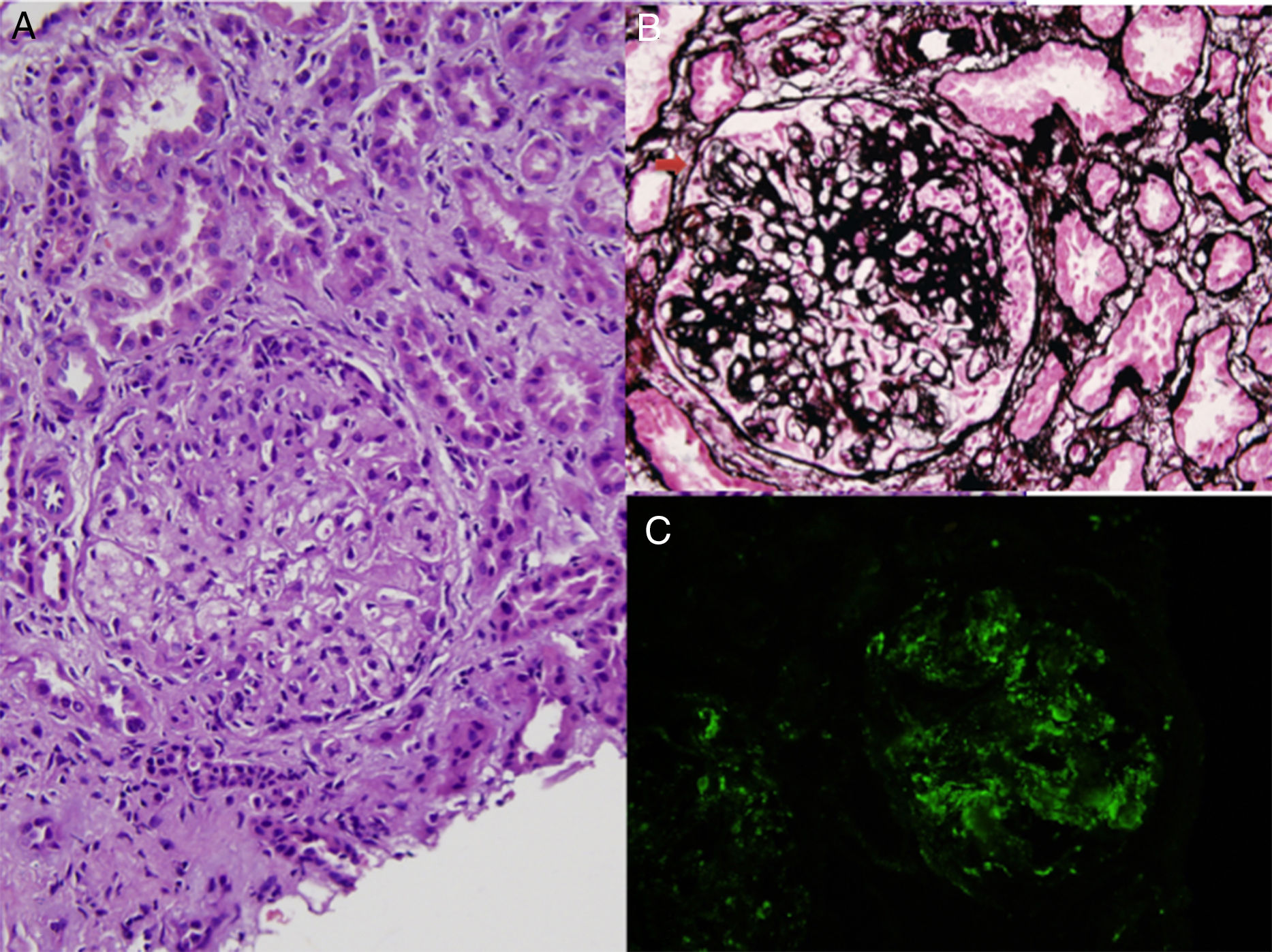
We present a case report that initially diagnosed as C3 glomerulopathy (C3G) and later developed aHUS treated with eculizumab that improved renal function.
A previously healthy 25-year-old woman presented with leg swelling. Only mild pretibial edema was detected in her physical examination. Laboratory workup revealed that an erythrocyte sedimentation rate 110mm/h, a serum creatinine concentration of 2mg/dL and albumin level of 2g/dL. Urinalysis revealed a proteinuria of 9g/day and microscopic hematuria.
Antinuclear antibodies, cytoplasmic antineutrophil cytoplasmic antibodies and perinuclear anti-neutrophil cytoplasmic antibodies were negative, serum C3 and C4 levels were normal. Kidney biopsy was performed. The biopsy contained 7 glomeruli which showed mesangial hypercellularity, segmental endocapillary proliferation, basement membrane thickening and duplication (Fig. 1A, B). Fibrocellular crescents were detected in 2 glomeruli (Fig. 1B). Focal segmental sclerosis was present in 40% of glomeruli. Immunofluorescence staining showed marked granular capillary wall and mesangial C3 deposition (Fig. 1C). Glomerular C1q and immunoglobulins were absent. The biopsy findings were consistent with C3G with a membranoproliferative pattern. Further classification of C3G could not be done, since electron microscopic examination was not available due to insufficient biopsy material. The patient was treated with 1mg/kg/day methylprednisolone and 1g/day mycophenolate mofetil (MMF), and previous treatment with ramipril was maintained.
Two months later, she was admitted to our clinic with shortness of breath with widely distributed crackles and ronchi on auscultation of both lungs. She had 3+pretibial pitting edema in both legs. Laboratory results showed renal failure (serum creatinine: 3.2mg/dL, uric acid: 8.2mg/dL, Na: 126mmol/L) with non-immune hemolytic anemia and thrombocytopenia. ADAMTS13 level was 30%. Atypical HUS was diagnosed and eculizumab treatment started after 5 sessions of plasmapheresis with hemodialysis because of persistent hypervolemia. Mycophenolate mofetil treatment was stopped because of thrombocytopenia. Genotyping of patient was performed with Sanger sequencing of CFH and CFI genes. Homozygosity for single-nucleotide polymorphism (SNPs) rs2298749 on 6 exon of CFI [S268S (TCG>TCA)] was detected. Hemodialysis was continued three times a week. After eight months of eculizumab therapy her serum creatinine levels were decreased to 3–4mg/dL and her hemodialysis was further reduced to once a week.
C3 glomerulopathy is defined as the presence of C3 deposits without immunoglobulins on immunofluorescence microscopy along with subendothelial and mesangial electron-dense deposits by electron microscopy. Electron microscopy is required to differentiate C3 glomerulonephritis from dense deposit disease.1 Although similarity between C3G and postinfectious glomerulonephritis is well known, we excluded postinfectious glomerulonephritis by clinical and follow-up data along with the presence of basement membrane changes by light microscopy.
Mutations in CFH, CFI and C3, and, the presence of anti-CFH antibodies have been previously demonstrated in patients with C3G and aHUS1 and based on these findings authors recommended that special diagnostic tests [C3NeF, serum factor H, complement factor H-related (CFHR) protein gene mutations, serum factor B, serum factor I, and membrane cofactor protein (MCP or CD46), soluble C5b-9, complement factor H-related (CFHR) protein gene mutations] should be obtained in patients with DDD or C3G.1–3 In our patient, we found a single nucleotide polymorphism in CFI gene; this SNP is not a disease-associated mutation but may cause susceptibility to these diseases.
To our knowledge, there is no prospective randomized trial on the treatment of C3G yet. Treatment suggestions are based on recent KDIGO meeting report.1 In the present case, we also started MMF plus steroid regimen however we stopped MMF earlier because of persistent thrombocytopenia.
Eculizumab is a humanized monoclonal antibody that binds with high affinity to C5 and has been approved by FDA and EMA for the treatment of aHUS. Eculizumab prevents cleavage of C5, thereby precluding formation of C5a and the terminal complement complex (C5b-9), which has been implicated in the pathogenesis of both DDD and C3G.4–6 In our case, eculizumab treatment resulted improvement of her residual renal function. Eculizumab is a potent drug that can act even though in stage 5 kidney failure. Although re-biopsy could not be performed because of thrombocytopenia, treatment with eculizumab improved her renal function, including with long standing fibrotic changes.
In conclusion, we present a patient who was initially admitted with nephritic syndrome and C3G was diagnosed and immunosuppressive treatment was initiated, two months later after diagnosis she was re-admitted with thrombotic microangiopathy and aHUS was diagnosed. After administration of eight month of eculizumab therapy her residual renal clearance improved and her dialysis treatment was further reduced to once a week. The present case report demonstrated a CFI genetic variation associated alternative pathway dysregulation causing C3G and aHUS in the same patient and highlighted the shared pathogenesis in these alternative complement pathway associated diseases.
The authors declare that they have no sources of funding for this study, and they have no conflicts of interest to declare.