Cardiovascular disease is the leading cause of the death in dialysis patients. Arteriovenous fistulas (AVFs) are associated with lower mortality and are viewed as the desired access option in most patients with advanced kidney disease needing dialysis. However, AVFs have significant and potentially deleterious effects on cardiac functions particularly in the setting of preexisting heart disease. This article provides a comprehensive and contemporary review to what is known about the impact of AVFs on: congestive heart failure, left ventricular hypertrophy, pulmonary hypertension, right ventricular dysfunction, coronary artery disease and valvular heart disease.
La enfermedad cardiovascular es la principal causa de muerte en los pacientes dializados. Las fístulas arteriovenosas (FAV) se asocian a una menor mortalidad y se consideran la opción preferible de vía de acceso en la mayor parte de los pacientes con enfermedad renal avanzada que requieren diálisis. Sin embargo, las FAV tienen efectos importantes y potencialmente nocivos sobre las funciones cardíacas, en especial en presencia de una cardiopatía preexistente. En este artículo se presenta una revisión completa y actualizada de los conocimientos existentes sobre las repercusiones que tienen las FAV en los trastornos de: insuficiencia cardiaca congestiva, hipertrofia ventricular izquierda, hipertensión pulmonar, disfunción ventricular derecha, enfermedad coronaria y valvulopatías cardíacas.
Cardiovascular disease is the leading cause of the death in patients receiving chronic renal replacement therapy.1–3 Arteriovenous fistulas (AVFs) have superior longevity, lower infection and mortality rates and are associated with lower cost, and hence have become the vascular access of choice for patients needing dialysis.4 Indeed, the prevalence of AVFs in the United States increased from 32% of all dialysis access in 2003 to 61% in 2012.5,6 Despite their association with a lower mortality, AVFs have significant effects on cardiac functions predominantly related to the increase in preload and cardiac output (CO). This article reviews the potential effects of the creation and the ligation of AVFs on cardiac function and their mechanisms.
It should be emphasized, at the outset, that determining the exact effects of AVFs on cardiac functions is fraught with problems for a couple of reasons: patients with end stage renal disease (ESRD) requiring dialysis almost invariably have volume overload due to water and salt retention. They also have pressure load due to arterial sclerosis and hypertension, and increased CO secondary to chronic anemia. In addition, many hemodialysis patients have significant pre-existing myocardial, valvular or coronary heart disease. It is, therefore, often difficult to tease out the exact contribution of an AVF to cardiac dysfunction in hemodialysis patients. Nevertheless, worsening in cardiac functions soon after AVF creation has been observed favoring a causative effect of the AVF on certain cardiac functions. The current literature suggests that the creation of AVF can cause or exacerbate the following conditions: congestive heart failure, left ventricular hypertrophy, pulmonary hypertension, right ventricular dysfunction, coronary artery disease, and valvular dysfunction.
AVFs and congestive heart failureCongestive heart failure (CHF) is highly prevalent among patients with ESRD. Approximately 35–40% of patients with ESRD have an established CHF diagnosis at initiation of hemodialysis.1,3,7–9 Patients with ESRD and CHF have a far worse prognosis than those without CHF.3,10 Since hemodynamic optimization is the corner stone of managing patients with ESRD as well as those with CHF, studying the hemodynamic effects of AVFs in patients with ESRD with and without CHF is a sensible task.
Long before we utilized AVFs for hemodialysis access, the hemodynamic effects of AVFs were studied in patients who developed AVFs secondary to trauma AVFs. In these patients, the development of an AVF was noted to be associated with an apparent increase in CO.11,12 The introduction of the ‘man-made’ AVFs for hemodialysis access provided more insight into the hemodynamic effects of these fistulas: First, the creation of an AVF leads to shunting of blood flow from the high resistance arterial system into the low resistance venous system, with a subsequent rise in venous return and CO.13 Second, the presence of an AVF decreases arterial impedance and thus lessens the left ventricular afterload. The lowering of arterial impendence may also reduce the effective circulating volume of the systemic circulation, activating arterial baroreceptors, and leading to secondary increase in cardiac sympathetic tone, contractility, and CO.14–16 The net effect of AVFs is a significant increase in CO.
Many studies investigated the impact of AVFs on echocardiographic indices of cardiac morphology and function.13,14,16–21 These studies consistently showed an increase in LV end-diastolic dimension (LVEDD), contractility, stroke volume and CO within 7–10 days after the surgical construction of AVF.13,14,18 Diastolic filling parameters (E to A ratio) were also impaired, indicative of worsening diastolic functions. On average, the creation of an AVF increases CO by 15–20% and left ventricular end-diastolic pressure by 5–10%.16 Additionally, biomarkers secreted in response to hypervolemia such as atrial naturietic peptide (ANP) and brain naturietic peptide (BNP), are both substantially increased,13,14 suggesting the presence of an cardiac volume overload despite an optimal overall body volume status.
The impact of these physiological effects of AVF on the cardiac function is controversial. While many studies suggested that the decreased vascular resistance and the increased CO are predisposing factors for the development or the worsening of heart failure,9 others suggested that the decrease in peripheral resistance and blood pressure with a parallel increase in ejection fraction could be potentially beneficial.22
Risk of worsening heart failure after AVFThere is no standard definition for high output CHF. The literature is inconclusive with regards to the incidence of worsening CHF after AVF creation. Most authors believe that the incidence of high output CHF among hemodialysis patients with AVFs is low, and that most patients with ESRD tolerate AVFs.23,24 This belief is supported by the fact that the literature on high output CHF in ESRD patients is limited to case reports and small series25–28 and that corrective measures (AVF banding or surgical ligation) due to AVF-related cardiac derangement are uncommon. Dixon et al. noted that the rate of AVF banding due to worsening CHF in a cohort of 204 patients (322 accesses) was only 2.6%.29 On the other hand, some authors suggest that high output CHF is not uncommon but is often overlooked.26,30 These authors argue that when cardiac deterioration occur in hemodialysis patients, it is usually attributed to the many risk factors that are highly prevalent in this population, and that the exact contribution of AVF to the worsening in cardiac functions is often not carefully sought.1,3,7–9 This is especially in patients with a long-standing AVFs, although AVF-related worsening CHF has been reported up to 10 years after AVF formation.31
It should be emphasized that most ESRD patients tolerate AVF well. However, given the deleterious outcomes in those who do not tolerate AVF, substantial efforts have been made to identify that small fraction of patients who are at risk for cardiac decompensation and high output CHF. There is compelling evidence that the development of high output CHF in ESRD patients with prior clinical or subclinical heart failure, is proportional to the vascular access flow (Qa),29,32,33 Most cases of high output CHF were reported in patients with Qa>2l/min.32 Upper arm (brachiocephalic) AVFs have twice the flow or lower arm (radiocephalic) AVFs.34 Macrae et al. noted that the average Qa in upper arm AVFs among ESRD patients enrolled in several studies was 1.13–1.72l/min.33 In the same cohort, 15% of patients had a Qa>2l/min. The ratio of access flow (Qa) to CO can be also be used to predict the risk of worsening CHF. A functional upper arm AVF has an average Qa/CO of 22%. Based on anecdotal experience, high output heart failure was associated with a Qa/CO of >40% in most cases.33 However, from an epidemiological point of view, high Qa is not clearly associated with an increase in mortality. In an interesting study by Al-Ghonaim et al., there was no increased risk of death at higher levels of Qa.35
Risk of developing de novo heart failure after AVFAlthough some authors postulate that cardiac decompensation in ESRD patients with AVFs occurs only in individuals with previously established chronic heart disease, there is evidence that AVF creation is a major risk factor for developing a new onset CHF.3,26,33,36 In the HEMO study, symptoms of CHF developed de novo during dialysis therapy in 17% of the patients.3 Albeit this could be purely due to the high prevalence of risk factors for developing CHF in the ESRD population, an independent effect of AVFs has been suggested. In an observational study of 562 pre-dialysis patients, the creation of AVF was more predictive of the development of decompensated CHF than a history of established CHF (odd ratio: 9.54 vs. 2.52, respectively).36 The median time between the creation of the AVF and the first episode of CHF was 51 days (range: 26–138). The location of AVF was, expectantly, closely related to the incidence of new CHF (40% in brachio-cephalic vs. 8% in radio-cephalic AVF).
AVFs and left ventricular hypertrophyLeft ventricular (LV) hypertrophy is highly prevalent among patients with ESRD, and is a strong predictor of morbidity and mortality.37,38 Although it is mainly a result of chronic systemic hypertension, volume overload and anemia, the presence of an AVF has a non-negligible effect on LV hypertrophy.38 Arteriovenous fistulas increase CO and lead to significant increases in both left ventricular wall mass and diameter in the long-term.13,20,39,40 Furthermore, LV hypertrophy tends to persist in patients who had successful kidney transplantation (KT) but have a remaining functional AVF.37,38 Closure of AVFs post-transplant has been shown to be associated with significant regression of LV hypertrophy, despite the observed post-closure increase in both systolic and diastolic blood pressure.17,19,41,42 This regression in LV mass starts as early as 3–10 weeks after fistula closure and becomes more pronounced at intermediate and long-term follow up.17,42 Although it is intuitive to speculate that regression of LV hypertrophy will lead to fewer cardiovascular events, a direct beneficial effect has not been proven. In fact, some authors believe that the potential benefit of such regression in LV mass after fistula closure might be blunted by the observed shift from a predominately eccentric hypertrophy to a predominantly concentric hypertrophy, a pattern that is known to be associated with worse long term outcomes.17,19
AVFs and pulmonary hypertensionPulmonary hypertension (PH) complicates ESRD with a prevalence of 12–45%.24,43 The presence of PH in the dialysis population confers 2–3 folds increase in all-cause mortality.43–45 In the majority of these patients, PH is post-capillary (pulmonary venous hypertension – World Health Organization Class II).46 Patients on hemodialysis have several risk factors for developing PH: LV systolic and diastolic dysfunction, volume overload, endothelial dysfunction and sleep-discorded breathing.15 However, the presence of an AVF has been shown to be an independent risk factor for the development of PH in ESRD patients.23,43,47,48 Paneni et al. compared echocardiography-derived peak systolic pulmonary artery pressure (PAP) between patients undergoing peritoneal dialysis (PD), and those receiving hemodialysis with radial and brachial AVFs. Systolic PAP was 29.7±6.7, 37.9±6.7 and 40.8±6.6mm Hg, respectively (p<0.001).47 In concordance with these findings, several other studies found a much less prevalence of PH in patients receiving PD compared to matched cohorts of patients undergoing hemodialysis via AVF.23,43,48,49 The mechanisms of AVF-related PH deserve more scrutiny.
The effects of volume overloadAs discussed previously, AVFs lead to decreased systemic vascular resistances, increased venous return, and therefore increased pulmonary blood and enhanced CO setting the stage for load-related PH.13,14,17,18,20 This theory has been supported by several studies that demonstrated a temporal relationship between PAP rise and AVF creation.49–52 These studies also showed a significant association between both the duration of AVF and the fistula flow with the severity of PH.49,50,52 Upper arm AVFs, known to have higher flow than lower arm AVFs, are associated with higher risk of developing PH.47,50 Compression of the AVFs by a sphygmomanometer,43,53 and surgical closure of the AVFs,54 both induce a rapid decrease in CO followed by a stable decrease in PAP. The late drop in PAP could be more pronounced than the initial drop when manual compression of an AVF is undertaken. In a case series of five patients in whom manual compression of the AVF in the catheterization laboratory was performed to predict a successful surgical closure, the chronic drop in CO following surgical AVF closure was 4 fold greater than the fall in CO seen during acute manual compression of the fistula (F. Raza, personal communication). Contrary to these findings, no association between the presence of AVFs or fistula flow and PAP was found in two small studies.23,24
Although AVF-associated volume overload seems to be the prime mechanism in development of PH in the hemodialysis population, the ability of an AVF alone to cause PH has been questioned.43 The pulmonary circuit has an enormous capacity and is usually able to tolerate significant volume loads before it decompensates. Hence, it has been proposed that in patients who develop PH after AVF creation, a baseline pulmonary vascular dysfunction is present leading to failure of the pulmonary circuit to accommodate the AVF-mediated elevated CO.43 This assumption is supported by two observations: (1) Patients without kidney disease or other significant co-morbidities are able to tolerate traumatic AVFs for a long time before they develop symptoms of PH or heart failure.55–58 (2) Kidney transplantation may revert PAP to normal in patients who still have a functioning AVF.53
Endothelial dysfunction has been suggested as an alternative or additive etiology for the development of pulmonary hypertension in patients with AVF. The vascular endothelium has complex and important physiological functions including controlling the vascular tone.59,60 Several studies have shown that patients with ESRD have impaired nitric oxide (NO) production,61 and increased endothelin-1 (ET-1) activity62 both of which have been implicated in the pathophysiology of PH.63 The impaired NO production in dialysis patients is thought to be secondary to the reduced bioavailability of NO substrate l-arginine, and the accumulation of endogenous inhibitors of NO synthase.64 The lack of the vasodilator properties of NO could contribute to an increased vascular tone and eventually to the causation of PH. Another potential cause of endothelial dysfunction in dialysis patients is the vascular wall shear stress associated with hemodialysis-related abnormal hemodynamics.62,65 In non-dialysis patients with chronic left to right blood shunts (e.g. patients with congenital heart disease), blood shunting augments wall shear stress which leads to endothelial damage, vascular remodeling and PH.65,66 Similar effects can be suggested in hemodialysis patients with functional AVFs.62,67
AVFs and right ventricular dysfunctionThe prevalence and pathophysiology of PH in patients on hemodialysis has been extensively studied. However, data on the development of right ventricular (RV) dysfunction in ESRD patients with AVF is scarce.
A few recent studies examined the effect of AVFs on echocardiographic parameters of RV dysfunction.47,68,69 Paneni et al. studied the prevalence of RV dysfunction in 94 patients on hemodialysis and 26 patients on PD.47 In this study, Tissue Doppler-derived myocardial performance index (MPI) was used as an indicator of global RV function. Myocardial performance index has been found to be more sensitive and less load-dependent than other echo indices in predicting RV dysfunction and adverse clinical outcomes.70,71 Right ventricular ejection fraction was preserved in the majority of patients across all subgroups. Right ventricular dysfunction (defined with an MPI>0.53) was, however, more prevalent in hemodialysis patients compared with PD patients (71.3 vs. 34.6%, p<0.001). The prevalence of RV dysfunction further increased in patients with brachial AVF compared with the radial access (90.6% vs. 61.3%, p<0.001). Logistic regression analysis adjusting for confounding factors including PAP showed that patients carrying AVFs displayed an increased risk of RV dysfunction when compared to the PD group [OR: 6.3 (95% CI: 2.0–19.5), p<0.001]. Again, the risk of RV dysfunction was further enhanced in patients with brachial AVF compared to those with the radial fistulas [OR: 5.9 (95% CI: 1.5–23.1), p<0.05]. In another study of 41 HD patients with AVFs, RV dysfunction (defined by an MPI of >0.55), was present in 18 patients (44%).68 In keeping with the findings by Paneni et al., the presence of AVF was associated with RV dysfunction independent of PAP values. DiLullo et al. also demonstrated that AVFs were associated with impaired RV systolic function (assessed by tricuspid annular plane excursion – TAPSE) and significant RV chamber dilatation compared to those dialyzed via central venous catheters.69
The presence of RV dysfunction independent of PAP values, argues against a major role for PH in the development of RV dysfunction in ESRD patients.47,68 It also suggests that AVF-dependent volume overload may by itself play a major role in triggering RV dysfunction in patients undergoing hemodialysis.47
AVFs and coronary artery diseaseSignificant coronary artery disease (CAD) is found in 30–40% of ESRD patients on hemodialysis.2,9,72 Compared with non-dialysis CAD patients, those on hemodialysis have substantially higher cardiac mortality, and poorer outcomes when undergoing percutaneous or surgical revascularization.73,74
The concern with AVFs in patients with CAD is three-fold: (1) the potential to provoke silent subendocardial myocardial ischemia due to increased oxygen demand and/or decrease oxygen supply. (2) The possible negative impact of AVFs on ipsilateral internal mammary artery (IMA) bypass graft, due to distal steal. (3) The interference of AVFs with cardiopulmonary bypass in patients undergoing coronary artery bypass (CABG) surgery.
Impact of AVF on coronary ischemiaIn dog studies, high-flow AVFs were associated with decrease subendocardial coronary perfusion mainly due to decreased diastolic pressure and shortening of the diastolic period.75,76 An interesting study by savage et al. used filtered non-fistula arm finger pressure recordings to examine the effects of AVFs on myocardial oxygen supply and demand surrogates.18 Diastolic pressure time index (DPTI), systolic pressure time index (SPTI), and the DPTI/SPTI ratio were used as indirect measures of myocardial supply, myocardial demand and subendocardial perfusion, respectively. The increase in oxygen demand due to the AVF-related increased CO was ameliorated by the decrease in oxygen demand due to the decreased peripheral vascular resistance caused by the AVF. The net effect on cardiac oxygen demand was neutral. However, cardiac oxygen supply and, therefore subendocardial perfusion, were both significantly reduced in patients with functioning AVFs. Manual compression of AVFs was associated with improved subendocardial perfusion surrogates.18,77 Despite the methodological limitations, these studies suggest a possible negative effect of AVF on subendocardial perfusion. However, confirmatory studies with invasive hemodynamic measurements or advanced imaging tools have not been reported.
Impact of AVF on coronary artery bypass grafting (CABG)In patients with ESRD who undergo hemodialysis via an upper extremity AVF ipsilateral to the internal mammary artery (IMA) used for CABG, both the bypass graft and the fistula are supplied by the subclavian artery. During hemodialysis, the AVF flow is significant, and can lead to shunting of blood away from the IMA graft (steal phenomenon). There are several reports of symptomatic IMA steal during dialysis session in patients with upper arm (brachio-cephalic) AVFs.78,79 Using echocardiography, Gaudino et al. elegantly demonstrated a significant decrease in IMA flow and hypokinesis of the anterior wall of the LV upon initiation of hemodialysis via an ipsilateral AVF in five patients. Both of these findings were reversed when the dialysis machine was turned off.80 Conversely, using similar methodology, two studies found that changes in the AVF flow did not significantly alter Doppler flow hemodynamics of either the ipsilateral or contralateral in situ IMA.81,82
The clinical impact of this potential ‘steal’ phenomenon on clinical outcomes was recently studied.83,84 Takami et al. retrospectively compared outcomes of 155 hemodialysis patients whose left anterior descending artery (LAD) was revascularized with the IMA ipsilateral to the AVF (ipsilateral group) and those whose LAD was grafted with the IMA opposite to the fistula (contralateral group).84 The overall 5-year survival and cardiac event-free rates were 58% and 74% in the ipsilateral group vs. 65% and 68% in the contralateral group, respectively (p=0.90 and p=0.07). A similar study by Feldman et al. showed comparable survival rates by higher non-fatal cardiac events in the ipsilateral group compared with the contralateral group (81.2% vs. 64%, p=0.023).83 Despite the conflicting evidence, it seems reasonable avoid, when possible, using an IMA coronary artery bypass graft ipsilateral to the AVF. Similarly, placing AVF in a patient with a functioning IMA would be better performed on the contralateral upper extremity.
Another cause for concern in hemodialysis patients with AVFs who are undergoing CABG is the possible interference of AVFs with the integrity of the cardiopulmonary bypass circuit. The excessive venous return to the heart due to high-flow AVFs, can compromise the myocardial protection offered by cardiopulmonary bypass, and lead to impromptu alterations in the surgical plan. In one case, the AVF had to be tied off after CABG to allow successful weaning of cardiopulmonary bypass.85 In another case, selective bicaval cannulation was needed to prevent cardiac distention due to the significant left to right shunting of blood via a functional AVF.86
AVFs and valvular heart diseaseValvular heart disease is common among patient on hemodialysis with a prevalence of 39–43%.3 The majority of valvular abnormalities (aortic stenosis, aortic regurgitation, mitral regurgitation and tricuspid regurgitation) are sensitive to volume overload. Despite that, data on the effects of AVF-associated volume load on patients with valvular heart disease is limited to patients with aortic stenosis (AS).87,88
Effects of AVF on aortic stenosisSignificant aortic stenosis is present in 3.3% of hemodialysis patients >65 years of age compared with 1–2% in the general population. Also, severe AS is rare in non-ESRD patients who are less than 50 but occurs in 3% of ESRD patients of similar age.89
There are two potential effects of AVFs on patients with severe AS: (1) the coexistence of AS and an AVF could complicate the assessment of the severity of aortic valve disease. Cardiac output has significant impact on the assessment of transaortic valve gradient in AS.90 In a patient with ESRD and suspected severe AS, manual compression of the AVF dropped the mean transaortic valve gradient from 45mmHg to 30mmHg.87 (2) The increase in CO associated with the creation of AVF can lead to acute or sub acute decompensation in patients with significant AS who had no symptoms or were minimally symptomatic prior to AVF surgery.88
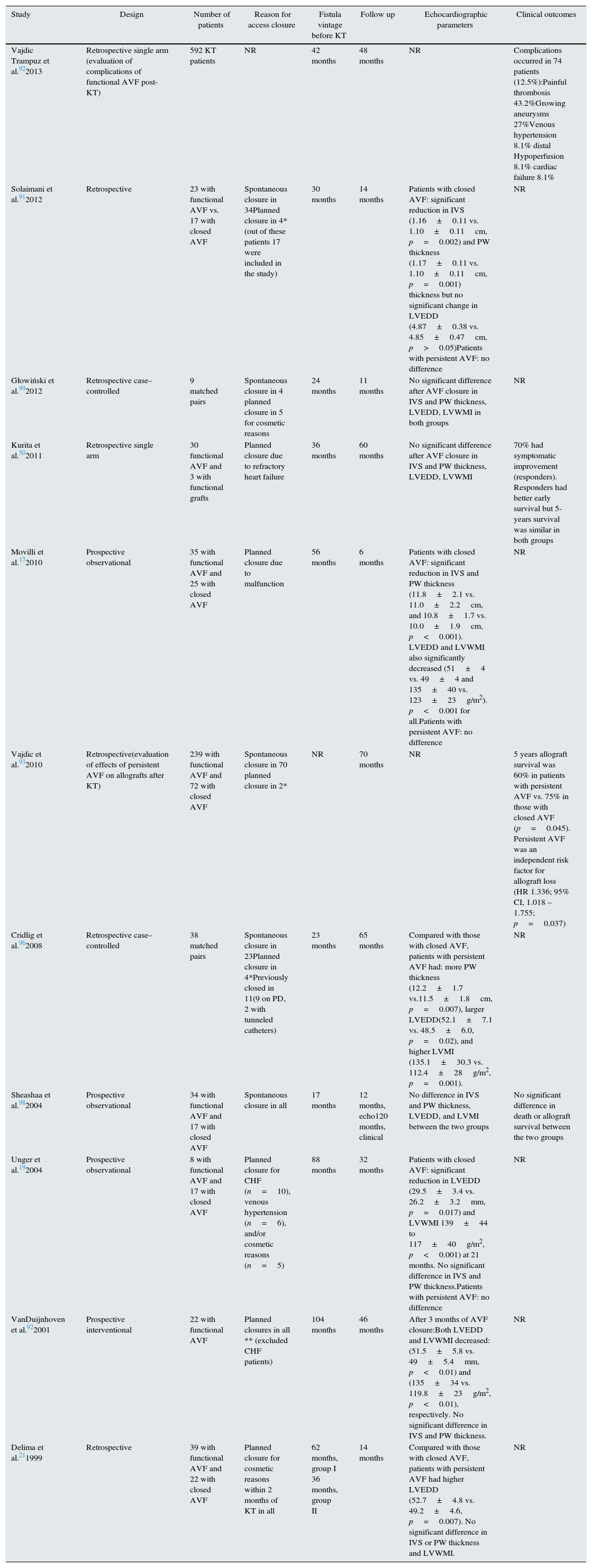
Is closing AVFs beneficial to the heart?Preserving dialysis access is a priority to both dialysis patients and their physicians. Closing AVFs in patients undergoing hemodialysis has been reserved for those with apparent access failure or apparent access-related complications.29 The management of AVFs in patients who underwent successful KT is a topic of ongoing debate.91–93 Most transplant physicians currently suggest that AVF closure is not routinely required in KT recipients with stable renal allograft function.91,94 Others believe that AVF closure is associated with significant beneficial effects on cardiac functions and on allograft survival.17,19,93,95,96 The benefit of AVF closure should be weighed against the small but the known potential life threatening complications associated with the closure procedure.97 A number of studies examined the effects of spontaneous or planned post-KT AVF closure on echocardiographic indices (LV wall mass index, wall thickness, and LVEDD). The potential impact of fistula closure on clinical outcomes was also investigated in a smaller number of studies. A summary of these studies is provided in Table 1.
A summary of studies examining the outcomes of spontaneous and planned AVF closure in kidney transplant recipients.
| Study | Design | Number of patients | Reason for access closure | Fistula vintage before KT | Follow up | Echocardiographic parameters | Clinical outcomes |
|---|---|---|---|---|---|---|---|
| Vajdic Trampuz et al.922013 | Retrospective single arm (evaluation of complications of functional AVF post-KT) | 592 KT patients | NR | 42 months | 48 months | NR | Complications occurred in 74 patients (12.5%):Painful thrombosis 43.2%Growing aneurysms 27%Venous hypertension 8.1% distal Hypoperfusion 8.1% cardiac failure 8.1% |
| Solaimani et al.912012 | Retrospective | 23 with functional AVF vs. 17 with closed AVF | Spontaneous closure in 34Planned closure in 4*(out of these patients 17 were included in the study) | 30 months | 14 months | Patients with closed AVF: significant reduction in IVS (1.16±0.11 vs. 1.10±0.11cm, p=0.002) and PW thickness (1.17±0.11 vs. 1.10±0.11cm, p=0.001) thickness but no significant change in LVEDD (4.87±0.38 vs. 4.85±0.47cm, p>0.05)Patients with persistent AVF: no difference | NR |
| Głowiński et al.992012 | Retrospective case–controlled | 9 matched pairs | Spontaneous closure in 4 planned closure in 5 for cosmetic reasons | 24 months | 11 months | No significant difference after AVF closure in IVS and PW thickness, LVEDD, LVWMI in both groups | NR |
| Kurita et al.502011 | Retrospective single arm | 30 functional AVF and 3 with functional grafts | Planned closure due to refractory heart failure | 36 months | 60 months | No significant difference after AVF closure in IVS and PW thickness, LVEDD, LVWMI | 70% had symptomatic improvement (responders). Responders had better early survival but 5-years survival was similar in both groups |
| Movilli et al.172010 | Prospective observational | 35 with functional AVF and 25 with closed AVF | Planned closure due to malfunction | 56 months | 6 months | Patients with closed AVF: significant reduction in IVS and PW thickness (11.8±2.1 vs. 11.0±2.2cm, and 10.8±1.7 vs. 10.0±1.9cm, p<0.001). LVEDD and LVWMI also significantly decreased (51±4 vs. 49±4 and 135±40 vs. 123±23g/m2). p<0.001 for all.Patients with persistent AVF: no difference | NR |
| Vajdic et al.932010 | Retrospective(evaluation of effects of persistent AVF on allografts after KT) | 239 with functional AVF and 72 with closed AVF | Spontaneous closure in 70 planned closure in 2* | NR | 70 months | NR | 5 years allograft survival was 60% in patients with persistent AVF vs. 75% in those with closed AVF (p=0.045). Persistent AVF was an independent risk factor for allograft loss (HR 1.336; 95% CI, 1.018 –1.755; p=0.037) |
| Cridlig et al.962008 | Retrospective case–controlled | 38 matched pairs | Spontaneous closure in 23Planned closure in 4*Previously closed in 11(9 on PD, 2 with tunneled catheters) | 23 months | 65 months | Compared with those with closed AVF, patients with persistent AVF had: more PW thickness (12.2±1.7 vs.11.5±1.8cm, p=0.007), larger LVEDD(52.1±7.1 vs. 48.5±6.0, p=0.02), and higher LVMI (135.1±30.3 vs. 112.4±28g/m2, p=0.001). | NR |
| Sheashaa et al.982004 | Prospective observational | 34 with functional AVF and 17 with closed AVF | Spontaneous closure in all | 17 months | 12 months, echo120 months, clinical | No difference in IVS and PW thickness, LVEDD, and LVMI between the two groups | No significant difference in death or allograft survival between the two groups |
| Unger et al.192004 | Prospective observational | 8 with functional AVF and 17 with closed AVF | Planned closure for CHF (n=10), venous hypertension (n=6), and/or cosmetic reasons (n=5) | 88 months | 32 months | Patients with closed AVF: significant reduction in LVEDD (29.5±3.4 vs. 26.2±3.2mm, p=0.017) and LVWMI 139±44 to 117±40g/m2, p<0.001) at 21 months. No significant difference in IVS and PW thickness.Patients with persistent AVF: no difference | NR |
| VanDuijnhoven et al.922001 | Prospective interventional | 22 with functional AVF | Planned closures in all ** (excluded CHF patients) | 104 months | 46 months | After 3 months of AVF closure:Both LVEDD and LVWMI decreased: (51.5±5.8 vs. 49±5.4mm, p<0.01) and (135±34 vs. 119.8±23g/m2, p<0.01), respectively. No significant difference in IVS and PW thickness. | NR |
| Delima et al.211999 | Retrospective | 39 with functional AVF and 22 with closed AVF | Planned closure for cosmetic reasons within 2 months of KT in all | 62 months, group I 36 months, group II | 14 months | Compared with those with closed AVF, patients with persistent AVF had higher LVEDD (52.7±4.8 vs. 49.2±4.6, p=0.007). No significant difference in IVS or PW thickness and LVWMI. | NR |
Spontaneous closure of AVFs (due to thrombosis), can result in a significant reduction in CO, LV wall mass index (LVMI), and LVEDD.96 Similar benefits were observed in patients who underwent planned surgical AVF closure due to graft dysfunction,17 cardiovascular deragments,19 or as part of a research protocol.41 Contrary to these findings, other studies found no significant effects of spontaneous or planned AVF closure on echocardiographic indices.21,91,94,98
Effects on clinical outcomesIn a large cohort of patients who underwent KT, the incidence of AVF-related complications requiring an intervention was 12.5% at 4 years.92 In these patients, cardiac decompensation and distal arm hypoperfusion constituted 16.2% of all complications. Transplant allograft survival was positively affected by AVF closure in one study,93 but was not different in another.98 In all-comers, AVF closure was not associated with a mortality benefit at 10 years.98 Only one study explored the effects of AVF closure on clinical outcomes in patients with refractory heart failure.95 In this study 30 patients with AVF and 3 with arteriovenous grafts were referred for access closure due to refractory CHF. There was an immediate significant improvement in CHF symptoms in 70% of patients (responders), but no benefit was seen in the other 30% (non-responders). The non-responders had higher prevalence of ischemic heart disease and longer durations since their AVF creation. Those who responded had better survival at 1 year, but had similar mortality rates as the non-responders at 5 years.
ConclusionThere are currently near 400,000 patients on hemodialysis and 180,000 kidney transplant recipients in the United States.6 The majorities of these patients have cardiovascular disease and have functional AVFs.1,2,5 Arteriovenous fistulas are associated with lower mortality and are viewed as the desired access option in most patients with ESRD needing dialysis.4 Arteriovenous fistulas are well tolerated by most patients. However, the potentially deleterious effects of AVFs, particularly in the setting of preexisting heart disease should not be underestimated. A multidisciplinary evaluation of patients with known heart disease before AVF creation is warranted. Additionally, in patients who develop dyspnea, heart failure, or pulmonary hypertension, AVF revision should be considered as an important therapeutic option, especially in those who underwent successful kidney transplantation.
Conflict of interestThe authors declare no conflict of interest.