The topic of what is the ideal calcium concentration in dialysis fluid (DF) is currently on the agenda of most scientific dialysis meetings. With a calcium concentrations of 1.25mmol/L, haemodialysis tolerance worsens, and with calcium levels of 1.5mmol/L, positive calcium balance together with the development of alkalemia at the end of the hemodialysis session may favour vascular calcification. Dealing with this issue is not easy because the concept of “balance” (concentration measured before and after the haemodialysis session) is interpreted as the theoretical “gradient” that is not measured nor calculated. The reason for this letter is to try to clarify these concepts and to provide some data.
Calcemia usually increases significantly during the haemodialysis session using acetate DF with a calcium concentration of 1.5mmol/L, this is more evident if the initial serum calcium concentration is low.1,2 If the acetate of the DF is replaced by citrate, the increase in the concentration of both total and ionic serum calcium after hemodialysis or online haemodiafiltration (OL-HDF) is less or or even null.3–5 It could be thought that by using a DF with 1.5mmol/L of calcium, the smaller increase in calcaemia at the end of the citrate session generate a positive calcium gradient from the DF to the blood, which would induce a positive balance for the patient, which would cause the patient's calcification; or, it could be interpreted that the smaller increase in calcemia observed with citrate is the result of a lower, or even negative, calcium balance.
The work of Steckiph6 compares the calcium balance in OL-HDF using a DF with citrate vs acetate, both with the same calcium concentration. It was a cross-sectional, randomized study in 18 patients; calcium and citrate balance were measured in the entire collection of DF. The calcium concentration in DF during the 2 periods was the same: 1.5mmol/L. The result was that the mean calcium balance at the end of OL-HDF was −274 (260) mg with DF containing citrate and +125 (174) mg with acetate DF.
So what is the true calcium gradient in the dialyser? The gradient is not the same across all capillaries of the dialyser and changes during the dialysis session. Further, the gradient is established as a function of diffusible calcium, which includes the ionized calcium and the bound calcium, which is essentially bound to bicarbonate and citrate present in both DF and blood.
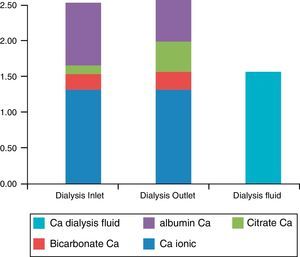
With DF containing citrate, the blood entering a dialyser capillar at the beginning of the session has a low concentration of both citrate and calcium-citrate complexes (Fig. 1). At the end of the capillar, the citrate concentration increased from 0.1 to 1mmol/L and the calcium -citrate complexes are up to 0.3–0.4mmol/L7 since citrate is being transferred from the DF. This calcium that is now bound to the citrate comes mainly from that bound to albumin, but also from free ionic calcium. At the end of the dialyser, the total blood-diffusible calcium has increased. This is not the case with the DF with acetate, which has a much lower affinity for calcium than citrate.
Concentrations of calcium in the blood (mmol/L) entering and leaving the dialyser, with the different components. Ionized calcium plus that bound to citrate and bicarbonate form the diffusible calcium in blood; all DF calcium is diffusible. Note that the concentration of blood-diffusible calcium is greater than that of the DF leaving the dialyser.
The concentration of calcium in the DF is different from the theoretical concentration. Table 1 shows the actual measurements of calcium in the DF with both citrate and acetate; the total calcium concentration is 0.16mg/dL (0.04mmol/L) less than the theoretical concentration. This is explained by the fact that, in the hydraulic circuit of haemodialysis machines, there is always a certain degree of microprecipitation, which is more pronounced if both bicarbonate and calcium levels are increased. The DF with citrate has a significantly lower ionic calcium concentration, due to the calcium-citrate complexes; therefore, the greater concentration of diffusible calcium in the blood and the lower concentration in the DF explain a lower Calcium balance with citrate than with acetate, as demonstrated by those who have measured it.6
Total calcium and ionic calcium concentrations measured in the dialysis fluid at the dialyser entry point.
| No.° | Average Ca++ (mmol/L) (DE) | Average total Ca (mg/dL) (DE) | Theoretical Ca in concentrate (mg/dL) | Total Ca – theoretical Ca (mg/dL) | |
|---|---|---|---|---|---|
| Citrate DF | 7 | 1.05 (0.156) | 6.34 (0.097) | 6.5 | −0.16 |
| Acetate DF | 13 | 1.22 (0.102) | 5.84 (0.18) | 6 | −0.16 |
| p | 0.04 | NS |
On 7 occasions, the DF contained 1mmol/L of citrate and 3.25mEq/L of calcium, and on 13, 3mmol/L of acetate and 3mEq/L of calcium. The same dialysis monitors (AK200 Us (Baxter®)) were used.
It is difficult to know the “gradients” of calcium during haemodialysis. Changes in iPTH variations during the dialysis session is an indirect manner to assess changes in calcaemia. In publications on DF with citrate,3–5 even with calcium concentrations of 1.65mmol/L, the iPTH increases or remains unchanged, but such changes in concentration may not be identical to the balance.
Thus, we believe calcium balances should be calculated using the entire DF, including the ultrafiltration. Baxter™ recommends increasing the calcium concentration in the DF with citrate by 0.15mmol/L compared to the DF with acetate in order to maintain the same balance.7 It could be that the DF with citrate with 1.5mmol/L is an intermediate solution between the 1.25 and 1.5mmol/L with acetate.
Please cite this article as: Pérez-García R, Albalate M, Sequera P, Ortega M. El balance de calcio es menor con un líquido de diálisis con citrato que con acetato. Nefrologia. 2017;37:109–110.