We present an unusual case of severe hypercalcemia of neonatal onset.
Patient of 4 days of life brought to the Emergency Department for constipation of 48h. Good oral tolerance and appetite. Hypotonic with good response to stimuli, without other symptoms. There is no family or personal background of interest.
Blood test with blood count, leukocyte formula and normal platelet levels; capillary gasometry and blood biochemistry with discrete metabolic acidosis: pH 7.33, bicarbonate 19.2 and ionic calcium 4.84mmol/l confirmed with serum calcium: 28.1mg/dl. Given the findings of severe hypercalcemia, enter the pediatric intensive care unit to complete the study.
On admission, figures of parathormone (PTH)>5000pg/ml were obtained (high limit of normality for age 78pg/ml), figures compatible with severe neonatal primary hyperparathyroidism. Normal thyroid activity. Noteworthy vitamin D deficiency (VitD): 16ng/ml; in probable relationship with maternal deficit.
In the radiological study, incipient osteopenia and cardiology showed a short corrected “Qt” interval, with rest of values and structurally normal heart.
Abdominal ultrasound showed evidence of right renal nephrocalcinosis without other findings; and the thyroid ultrasound showed no hyperplasia or glandular adenomas.
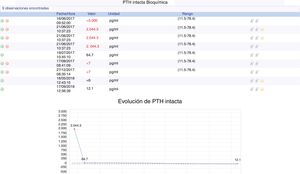
We performed thyroid scintigraphy that surprisingly objectified the physiological distribution of the tracer. In the presence of severe hypercalcemia due to suspicion of severe primary neonatal hyperparathyroidism (NSHPT), an urgent treatment is initiated with: abundant hydration (physiological saline at 100/m2/H); extrarenal purification therapy; furosemide (1mg/kg/day); systemic corticosteroid (methylprednisolone 2mg/kg/day); calcitonin (5UI/kg/day); and pamidronate (1mg/kg/day). Requires mechanical ventilation for the first 48h. This treatment is effective, with normalization of calcium values (2.43mmol/l of Ca ionic) in the first 48h and PTH level of decrease (after 5 days of treatment, 2044pg/ml) (Fig. 1).
After these results, treatment reduction was initiated, confirming a new increase in ionic calcium levels (maximum 2.1mmol/l and 11mg/dl), forcing it to restart.
At 12 days of age, she was transferred to a child ENT service where she underwent total parathyroidectomy, with intraoperative diagnostic confirmation of visu. They were removed 6 orthotopic parathyroid glands all of them, reimplanting one in the forearm, apparently not functioning. Objective to the contrary, in the last analytical control, figures of PTH in ascent (12.1pg/ml) that could show restart of activity, not enough for the moment for the adequate maintenance of phosphocalcic metabolism.
The reimplanted parathyroid glands may take up to restart their activity between 6 and 12 months, according to the literature described. However, we could consider the increase of this range of waiting to check the progressive rise and recovery in patients several months beyond the intervention, as in our case, after 16 months of the reimplantation.
The subsequent genetic study revealed a double mutation in heterozygosis of the “CaSR” gene. Diagnosing symptomatic severe neonatal hyperparathyroidism.
After the intervention, the patient presented progressive hypocalcemia, without renal losses; together hypophosphoremia and hypomagnesemia. Compatible with “hungry bone syndrome”, treatment is started with 1,25 hydroxyvitamin D3 (calcitriol, 0.50mcgr/day) and calcium supplements (calcium carbonate 1000mg/day orally and calcium gluconate 216mg/day orally, with total calcium contributions of 345mg/kg/day) together with phosphorus and magnesium.
Currently the patient is 15 months old, is asymptomatic and evolves favorably. The main sequelae is a permanent hypoparathyroidism with hypocalcemia and diffuse osteopenia, in maintenance treatment with calcium supplements, cholecalciferol and calcitriol.
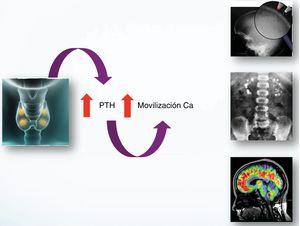
The presentation of severe neonatal hyperparathyroidism is diverse; from asymptomatic cases to respiratory symptoms, neurological, failure of the plan etc. A comprehensive differential diagnosis is necessary (Fig. 2).1
There is no “gold standard” diagnostic test and the imaging tests have proved ineffective, with a high false negative rate.
The treatment is fundamentally based on:
- •
The urgent and vital correction of high levels of hypercalcemia with hyperhydration, calcimimetics (cinacalcet), bisphosphonates (pamidronate), diuretics (furosemide) and corticosteroid therapy (methylprednisolone).
- •
Surgical etiological treatment.
- •
Treatment of subsequent sequelae: hypocalcemia and hungry bone syndrome; treated with 25OH-vitamin D3 (calcitriol/alfacalcidol) and calcium (calcium gluconate and carbonate).
The existence of a biological marker that determines the favorable response or not to medical treatment, would save unnecessary pharmacotherapy periods. However, there is currently no such parameter.1,2 Therefore, surgical treatment is of choice when adequate control with pharmacotherapy is not achieved, as in our case.
As for the genetic study, mutations in the CASR gene of the parathyroid cells are related to alteration of calcium homeostasis. This receptor records calcium levels in the blood. Its mutation causes the insensibility of these cells to the variations of calcemia, generating an increase in PTH and glandular hyperplasia. This mutation is observed in hyperplasia, adenomas and NSHPT.
In our patient, DNA amplification was carried out by PCR of the CASR gene, detecting two changes in heterozygosis. The c.73C>T mutation had already been previously described, however, the second mutation found; c.1981T>C, is “novo” and probably pathogenic.
More than half of cases of hyperparathyroidism in children reported in the literature show family history; in our patient, the study of progenitors, revealed absence of mutations in the father and c.73C>T mutation in the mother. Many authors defend that family screening could be effective and efficient in the control of the disease.3
In conclusion, NSHPT should be considered in the differential diagnosis of newborns with hypercalcemia. The initial medical treatment will be vital for the patient, determining the need or not of subsequent surgical treatment. It is essential to establish the genetic diagnosis as well as a lifelong medical follow-up.
Please cite this article as: Segura González M, Carrasco Hidalgo-Barquero MC, Hidalgo-Barquero del Rosal E. Calcio de 28mg/dl en paciente de 4días de vida. Tratamiento urgente y seguimiento posterior. Nefrologia. 2019;39:552–554.