There is a little information about of expression of C4d (complement fragment) in Focal segmental glomerulosclerosis (FSGS) subtypes. Our aim was to determine the expression of C4d in FSGS subtypes in percutaneous native renal biopsies in a second-level hospital and its correlation with clinical, biochemical and histological variables.
Material and methodsA retrospective study in paraffin blocks of patients with biopsy with FSGS aged 16–65 years, indistinct sex, not diabetic or obese. Immunohistochemistry was performed for C4d and their expression was analyzing in non-sclerosed glomerular capillaries (GC) and sclerosis areas (SA). Clinical and biochemical variables were recorded. The cases were divided into C4d positive and C4d negative groups and compared. The correlation between C4d staining scores in CG and SA with clinical and biochemical variables were analyzed.
ResultsTwenty samples were analyzed, 4 for each subtype. At the time of biopsy average age 38.8 ± 18.6 years, 65% male, 8.7% were hypertension. The percentage of positivity for C4d was 40% in GC, 30% SA and 35% in mesangium. The highest expression was for cellular and collapsing subtypes. C4d positivity cases had increased proteinuria (p = 0.035). A significant correlation was found between percentage of C4d expression in CG with SA (p = 0.012) and SA with tubular atrophy and interstitial fibrosis (p < 0.05).
ConclusionsC4d expression in FSGS predominated in the cellular and collapsing subtypes, which translates complement activation. C4d is a possible surrogate marker in GSFS.
Existe poca información acerca de la expresión del C4d (fragmento del complemento) en la glomeruloesclerosis focal y segmentaria (GEFS) y sus variantes. El objetivo del estudio fue determinar la expresión de C4d en las variantes de GEFS en biopsia renal percutánea (BRP) de riñones nativos en un hospital de segundo nivel y su correlación con variables clínicas, bioquímicas e histológicas.
Material y métodosEstudio retrospectivo en bloques de parafina de pacientes con BRP con GEFS de 16–65 años, sexo indistinto, no diabéticos ni obesos. Se realizó inmunohistoquímica para C4d, analizando su expresión en capilares glomerulares (CG) no esclerosados y áreas de esclerosis (EC). Se registraron variables clínicas y bioquímicas. Los casos fueron clasificados en C4d positivo o negativos y se compararon entre ellos. Se analizó la correlación entre las puntuaciones de C4d en CG y EC con variables clínicas y bioquímicas.
ResultadosSe analizaron 20 muestras, 4 para cada variante. Al momento de la BRP la edad fue de 38.8 ± 18.6 años, 65% sexo masculino, 8.7% hipertensos. El porcentaje de positividad para C4d fue 40% en CG, del 30% en EC y del 35% en mesangio. La mayor expresión de C4d fue para las variantes celular y colapsante. Los casos C4d positivos tenían mayor proteinuria (p = 0.035). Se encontró correlación entre el porcentaje de expresión de C4d en CG con EC (p = 0.012) y de EC con atrofia tubular y fibrosis intersticial (p < 0.05).
ConclusionesLa expresión de C4d en GFS predominó en la variante celular y colapsante por probable activación del complemento. C4d es un posible marcador subrogado en GEFS.
Focal segmental glomerulosclerosis (FSGS) is a renal lesion caused by various aetiologies and pathological processes causing damage and loss of the podocyte.1,2 FSGS lesions can be broadly subdivided into primary (idiopathic), genetic and secondary forms,2 and in practical terms, FSGS is classified as primary or secondary depending on whether a causal aetiology is identified.3 Patients with primary FSGS present with nephrotic syndrome, focal segmental lesions on light microscopy, undefined immune complex deposits on immunofluorescence, and generalised effacement of podocytes on electron microscopy.1
According to the Columbia classification, there are 5 variants for FSGS: classic, perihilar, cellular, tip and collapsing,4 each with a different prognosis in terms of evolution and reaching end-stage chronic kidney disease. In FSGS, IgM and C3 deposits are frequently found in sclerotic areas, and sometimes in the mesangium in non-sclerotic areas.5
C4d is a fragment of C4 produced during complement activation through the classical or lectin pathways.4,5 C4d is very stable and binds covalently to cell surfaces, where it can be detected by immunohistochemistry. The study of C4d in glomerulopathies has been of clinical interest in recent years, since the mesangial deposition of C4d can be used as a poor prognostic factor in IgA nephropathy.6
Our objective was to determine the expression of C4d in primary FSGS variants by percutaneous renal biopsy (PRB) of adult native kidneys evaluated in a secondary hospital of the Mexican Institute of Social Security and its correlation with clinical, biochemical and histological variables.
Material and methodsRetrospective cross-sectional study carried out in paraffin blocks of patients with PRB with a diagnosis of FSGS, aged between 16 and 65 years, gender indistinct, with complete pathology record and report (≥7 glomeruli, with optical microscopy and immunofluorescence), who were not diabetic or obese, found to be non-reactive to human immunodeficiency virus and hepatitis B and C. No history of cancer in the last 5 years, without previous treatment and/or steroid use before PRB.
PRB findings were classified according to Columbia criteria. The samples were evaluated for both C4d and variant corroboration by two pathologists who examined the biopsies separately, and differences in diagnosis between the two pathologists were resolved by consensus.
Detection of C4d by immunohistochemistry was performed on paraffin-fixed tissue in 3 μ sections in formaldehyde and the C4d polyclonal rabbit anti-human antibody (Cell Marque™, USA) catalog BSB 2836 was used, in accordance with the supplier's recommendations.
Any expression in glomerular capillaries (GC) without sclerosis was considered positive in accordance with van de Lest et al.7
Patients were classified as positive when they presented C4d deposits in any percentage in GC in those glomeruli without sclerosis,7 and as C4d-negative in their absence. Furthermore, the expression of C4d was evaluated in areas of sclerosis (AS) and mesangium. Clinical variables (age, hypertension, body mass index) and biochemical variables (haemoglobin, glucose, urea, creatinine, C3 and C4 complement, 24-h urine proteinuria) were recorded, and the estimated glomerular filtration rate (eGFR) was calculated using the 4-variable Modification of Diet in Renal Disease equation. Urinary protein excretion and serum creatinine levels at baseline were comparable between groups.
The statistical analysis was performed using the statistical software SPSS® v20.0 in Spanish (SPSS Inc., Chicago, IL, USA). Quantitative data were expressed as mean ± standard deviation or median with interquartile range according to their distribution. Differences in quantitative data were evaluated using Student's t-test or nonparametric test depending on the distribution of the data. The Pearson or Spearman correlation test was used depending on the normality of the data. Logistic regression analysis was performed between C4d expression and the variables serum creatinine, proteinuria and eGFR, as well as multinominal regression analysis between C4d expression and histological variants of FSGS. A p-value of <0.05 was considered significant.
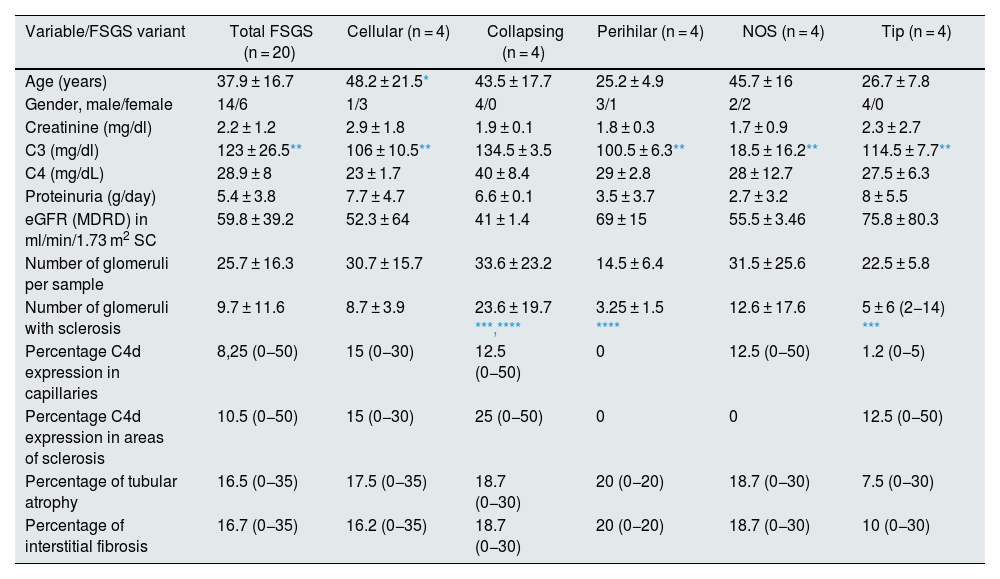
Results20 tissue samples with FSGS were analysed, there were 4 for each variant. At the time of the PRB, the mean age was 38.8 ± 18.6 years, 65% male, 8.7% hypertensive. The main indication for PRB was nephrotic syndrome in 60% of cases, followed by subnephrotic proteinuria in 20%. At the time of PRB, the average haemoglobin was 14.8 ± 2.9 g/dl, urea 93.3 ± 75.9 mg/dl, creatinine 1.9 ± 1.3 mg/dl, proteinuria 5.4 ± 4 g/day and serum albumin 2.4 ± 0.3 g/dl. The rest of the demographic, clinical and pathological data are shown in Table 1. The median eGFR was 68.5 (range 14–132.6) ml/min/1.73 m2. At the time of the kidney biopsy, 6 patients (30 %) showed a decrease in eGFR < 60 mL/min/1.73 m2.
Demographic, clinical and pathological data overall and for each variant.
| Variable/FSGS variant | Total FSGS (n = 20) | Cellular (n = 4) | Collapsing (n = 4) | Perihilar (n = 4) | NOS (n = 4) | Tip (n = 4) |
|---|---|---|---|---|---|---|
| Age (years) | 37.9 ± 16.7 | 48.2 ± 21.5* | 43.5 ± 17.7 | 25.2 ± 4.9 | 45.7 ± 16 | 26.7 ± 7.8 |
| Gender, male/female | 14/6 | 1/3 | 4/0 | 3/1 | 2/2 | 4/0 |
| Creatinine (mg/dl) | 2.2 ± 1.2 | 2.9 ± 1.8 | 1.9 ± 0.1 | 1.8 ± 0.3 | 1.7 ± 0.9 | 2.3 ± 2.7 |
| C3 (mg/dl) | 123 ± 26.5** | 106 ± 10.5** | 134.5 ± 3.5 | 100.5 ± 6.3** | 18.5 ± 16.2** | 114.5 ± 7.7** |
| C4 (mg/dL) | 28.9 ± 8 | 23 ± 1.7 | 40 ± 8.4 | 29 ± 2.8 | 28 ± 12.7 | 27.5 ± 6.3 |
| Proteinuria (g/day) | 5.4 ± 3.8 | 7.7 ± 4.7 | 6.6 ± 0.1 | 3.5 ± 3.7 | 2.7 ± 3.2 | 8 ± 5.5 |
| eGFR (MDRD) in ml/min/1.73 m2 SC | 59.8 ± 39.2 | 52.3 ± 64 | 41 ± 1.4 | 69 ± 15 | 55.5 ± 3.46 | 75.8 ± 80.3 |
| Number of glomeruli per sample | 25.7 ± 16.3 | 30.7 ± 15.7 | 33.6 ± 23.2 | 14.5 ± 6.4 | 31.5 ± 25.6 | 22.5 ± 5.8 |
| Number of glomeruli with sclerosis | 9.7 ± 11.6 | 8.7 ± 3.9 | 23.6 ± 19.7 ***,**** | 3.25 ± 1.5 **** | 12.6 ± 17.6 | 5 ± 6 (2−14) *** |
| Percentage C4d expression in capillaries | 8,25 (0−50) | 15 (0−30) | 12.5 (0−50) | 0 | 12.5 (0−50) | 1.2 (0−5) |
| Percentage C4d expression in areas of sclerosis | 10.5 (0−50) | 15 (0−30) | 25 (0−50) | 0 | 0 | 12.5 (0−50) |
| Percentage of tubular atrophy | 16.5 (0−35) | 17.5 (0−35) | 18.7 (0−30) | 20 (0−20) | 18.7 (0−30) | 7.5 (0−30) |
| Percentage of interstitial fibrosis | 16.7 (0−35) | 16.2 (0−35) | 18.7 (0−30) | 20 (0−20) | 18.7 (0−30) | 10 (0−30) |
Means ± standard deviations and percentages (minimum and maximum) are reported. Significant correlations are shown in bold.
MDRD: Modification of Diet in Renal Disease; BSA: body surface area; eGFR: estimated glomerular filtration rate.
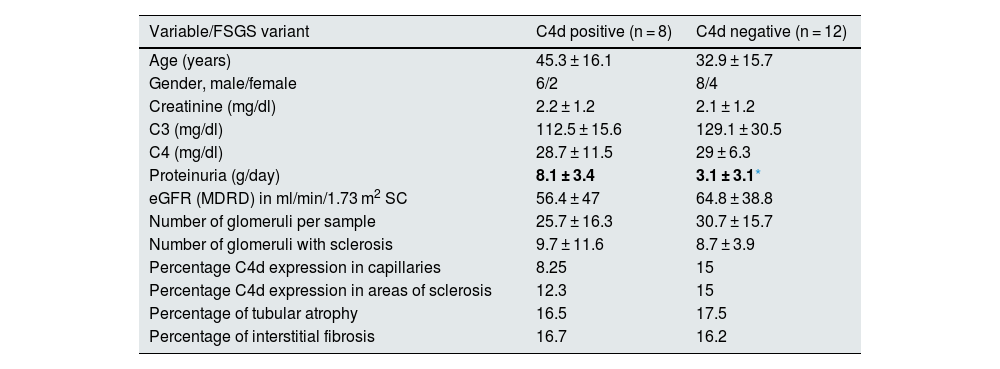
The percentage of positivity for C4d was 40% in GC, 30% in AS and 35% in mesangium. Thecomparison of the 8 C4d-positive samples versus the 12 negative ones, showed only a difference in the proteinuria per collected in 24 h (p = 0.035). The rest of the variables are shown in Table 2.
Demographic, clinical and pathological data between C4d positive and negative cases.
| Variable/FSGS variant | C4d positive (n = 8) | C4d negative (n = 12) |
|---|---|---|
| Age (years) | 45.3 ± 16.1 | 32.9 ± 15.7 |
| Gender, male/female | 6/2 | 8/4 |
| Creatinine (mg/dl) | 2.2 ± 1.2 | 2.1 ± 1.2 |
| C3 (mg/dl) | 112.5 ± 15.6 | 129.1 ± 30.5 |
| C4 (mg/dl) | 28.7 ± 11.5 | 29 ± 6.3 |
| Proteinuria (g/day) | 8.1 ± 3.4 | 3.1 ± 3.1* |
| eGFR (MDRD) in ml/min/1.73 m2 SC | 56.4 ± 47 | 64.8 ± 38.8 |
| Number of glomeruli per sample | 25.7 ± 16.3 | 30.7 ± 15.7 |
| Number of glomeruli with sclerosis | 9.7 ± 11.6 | 8.7 ± 3.9 |
| Percentage C4d expression in capillaries | 8.25 | 15 |
| Percentage C4d expression in areas of sclerosis | 12.3 | 15 |
| Percentage of tubular atrophy | 16.5 | 17.5 |
| Percentage of interstitial fibrosis | 16.7 | 16.2 |
FSGS: focal segmental glomerulosclerosis; MDRD: Modification of Diet in Renal Disease; BSA: body surface area; eGFR: estimated glomerular filtration rate.
No sample analysed showed joint positivity in the expression of C4d(−)/C1q(−)/C3(−); a 10% showed joint positivity of C4d(+)/C3(+)/C1q(−); 5% were C4d(−)/C3(+)/C1q(−). A 20% were C4d(+)/IgM(+); and finally, a 10% of the samples had positivity for C4d(+)/IgM(+)/C3(+).
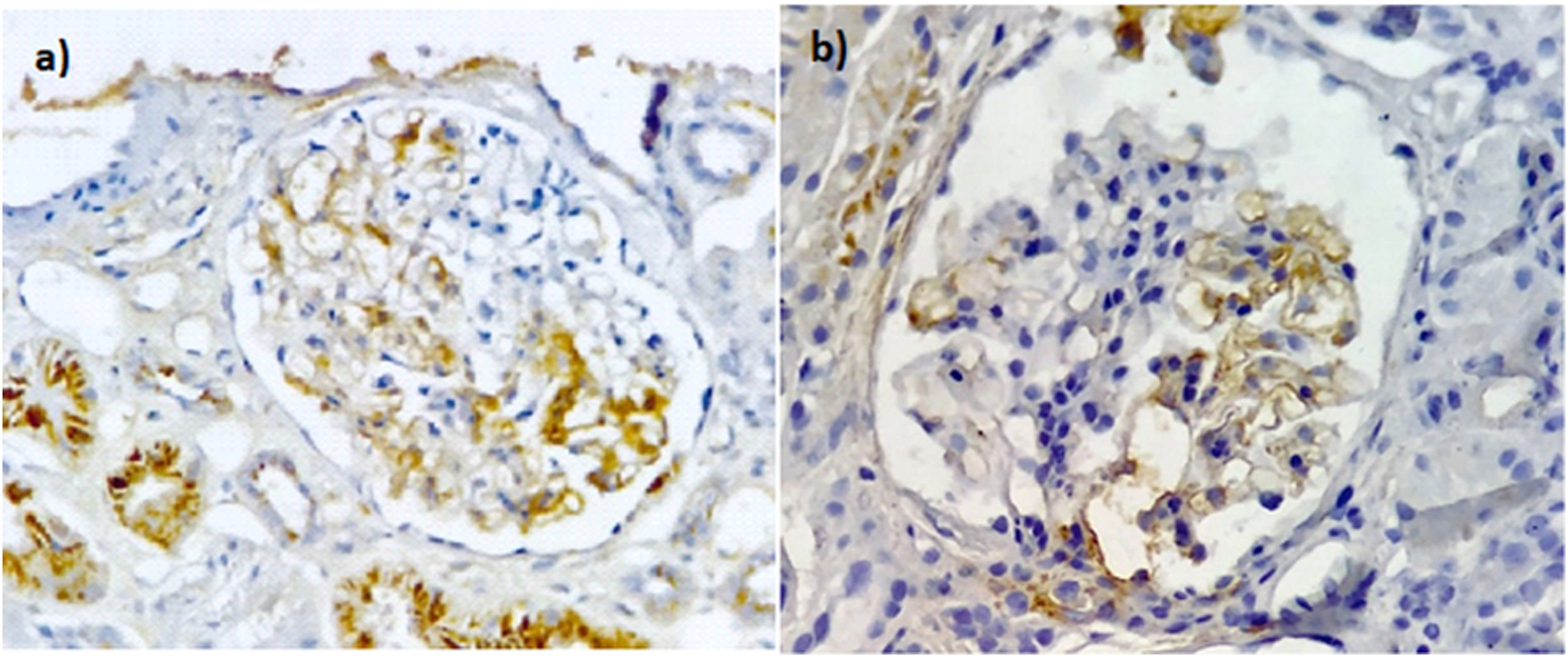
The positivity for the variants was: NOS — one in GC and one in AS; perihilar — none in GC and one in AS; cellular — 3 in GC and 3 in AS; tip — one in GC and one in AS; collapsing — 3 in GC and 2 in AS. Analysis of the percentage of expression between the variants showed no statistical differences in the expression of C4d (Fig. 1).
Immunoperoxidase staining with anti-C4d antibodies in patients with FSGS. (a) Segmental positive with moderate intensity in the mesangium and endothelial cells of the glomerular capillaries with a uniform granular distribution. Non-specific deposits in the cytoplasm of the tubules. (b) Segmental positive with moderate intensity in the mesangium and endothelial cells of the glomerular capillaries, also C4d without expression in the endothelium of the peritubular capillaries. Images ×40.
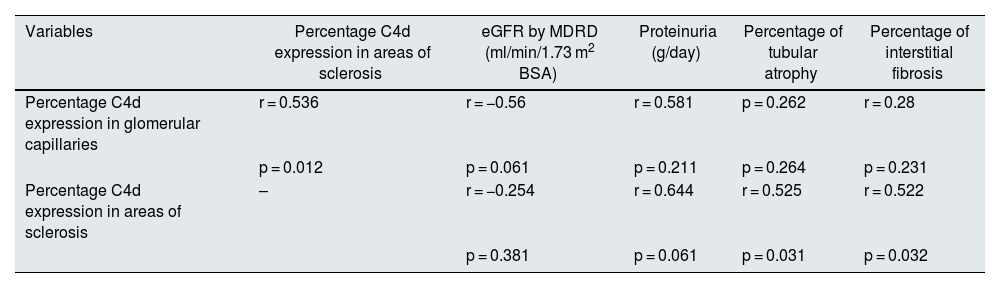
The correlation between C4d expression in GC and AS was significant, as well as for AS with tubular atrophy and interstitial fibrosis (Table 3); however, it was not significant for C3, C4, eGFR and 24-h proteinuria.
Pearson correlations between the percentage of C4d in glomerular capillaries, areas of sclerosis, eGFR, proteinuria, tubular atrophy and interstitial fibrosis in PRB with FSGS.
| Variables | Percentage C4d expression in areas of sclerosis | eGFR by MDRD (ml/min/1.73 m2 BSA) | Proteinuria (g/day) | Percentage of tubular atrophy | Percentage of interstitial fibrosis |
|---|---|---|---|---|---|
| Percentage C4d expression in glomerular capillaries | r = 0.536 | r = −0.56 | r = 0.581 | p = 0.262 | r = 0.28 |
| p = 0.012 | p = 0.061 | p = 0.211 | p = 0.264 | p = 0.231 | |
| Percentage C4d expression in areas of sclerosis | – | r = −0.254 | r = 0.644 | r = 0.525 | r = 0.522 |
| p = 0.381 | p = 0.061 | p = 0.031 | p = 0.032 |
Significant correlations are shown in bold.
PRB: percutaneous renal biopsy; FSGS: focal segmental glomerulosclerosis; MDRD: Modification of Diet in Renal Disease; BSA: body surface area; eGFR: estimated glomerular filtration rate.
A multinomial logistic regression model was used to analyse an association between C4d expression and FSGS variants, which was non-significant. Likewise, the linear regression model used to look for an association between C4d expression with the clinical variables eGFR and 24-h proteinuria was not significant.
DiscussionThere are few studies that analyse C4d expression in FSGS. We found that 40% of the samples analysed had positivity for C4d in GC, which is similar to the 42.9% positivity reported by Drachenberg et al.,8 but less than that reported by van de Lest et al.,7 who reported a prevalence of 73% of glomeruli positive for C4d in 40 patients who were older, had proteinuria and had received previous treatment, which could have influenced the difference in expression.
We found a correlation between C4d expression in GC and the proteinuria in 24-h urine, which supports the glomerular damage caused by C4d activation and is similar to other reports.9,10 Recently, Chebotareva et al.11 reported higher expression of some proteins in urine among patients with FSGS than in patients with membranous nephropathy. These proteins include some complements (C4b, C9, factor B and I) and a decrease in CD59, which shows that greater damage is associated with complement activation.
In our study, in 35% of the samples analysed with FSGS, C4d expression was found in the mesangium, similar to what was reported by Heybeli et al.12 This finding was previously reported in membranous nephropathy, which shows local activation of complement.6
In our study, the cellular and collapsing variants showed greater expression of C4d in GC, similar to what was reported by Drachenberg et al.8 and Huang et al.13; the latter reported greater activity in the cellular variants than in the tip and NOS variants.
A result worth highlighting is the positive correlation between the expression of C4d in GC and AS in 6 of the 20 samples analysed, which shows statistical significance and is similar to the findings of other authors.7,8 It should be borne in mind that previous studies report that these glomerular C4d deposits may precede the development of FSGS, which shows that complement activation may play a pathogenic role in its development.7
In kidney graft biopsies, C4d is deposited in the vascular endothelium, with the mesangium being the internal control. C4d has a covalent bond that binds to nearby cells where immune complexes are deposited and gives it a longer half-life, meaning that C4d serves as a marker of antibody-mediated tissue injury. Complement activation in FSGS is possibly through the classical and alternative pathways.5,13
In our study, none of the samples analysed showed positivity in the co-expression of C4d, C1q and C3, which, citing Gupta et al.,14 could be interpreted as no activity in the classic complement pathway. However, 10% showed joint positivity of C4d and C3 with negative C1q, which could be assumed as an activation of the lectin pathway (one sample was a collapsing variant and the another cellular). Only 5% showed positivity for C3 with C4d with negative C1q (a sample of the tip variant), which shows activation of the alternative complement pathway. However, we did not find a decrease or increase in serum complement concentrations of C3 and C4, although we did find differences in their concentrations in the NOS variant, which is different from what was reported by Huang et al.,13 who in a sample of 70 patients with primary FSGS reported an increase in serum and urinary concentrations of C3a, C5a and C5b-9. We must mention that it is a group with previous treatment, of which 61.7% showed relapse after remission. In this regard, Thurman et al.15 studied 19 patients with FSGS and reported that plasma and urinary levels of Ba (complement fragment) were positively correlated with proteinuria level, concluding that the complement system is activated in patients with primary FSGS and that elevated levels of Plasma Ba correlate with more severe disease.
Additionally, we found that 10% of the samples were positive for C4d, IgM and C3 with trapping in glomerular capillaries. This is due to the nature of IgM, which is a pentamer and therefore has a greater capacity to activate the complement than IgG.15
Zhang et al.16 showed unfavourable therapeutic responses and worse results in renal function in 58 patients with primary FSGS and IgM and C3 deposition, which indicates that IgM and C3 deposition could imply the progression of the disease through complement activation. However, Trachtman et al.17 show that natural IgM and complement do not initiate glomerular injury, but rather exacerbate the injury caused by other factors and contribute to the progression of the disease. Unlike other authors, we found no correlation between C4d expression in GC or AS with creatinine, eGFR and proteinuria.
Our study has some limitations, such as the fact that it was single-centre and the sample size. The diagnostic approach taken by the clinician, which led to the PRB and histopathological diagnosis, as well as the classification of the different variants, were prior to the publication of the new KDIGO 2021 guidelines,18 leaving FSGS responsible, regardless of the aetiology. Furthermore, it has the limitation of not having genetic or molecular FSGS tests on the lectin pathway and/or specific confirmatory tests for the alternative pathway.
However, it has the strength that patients with obesity, diabetes, steroid use and viral diseases were not included. We consider that more additional and prospective studies are required in the future to assess the possible clinical and prognostic significance of C4d expression in FSGS. It is likely that the approach to FSGS should be redirected as a disease more associated with autoimmune causes, with C4d deposition without hypocomplementemia.
ConclusionsThe expression of C4d in FSGS predominated in the cellular and collapsing variant in both GC and AS, which translates into complement activation in both the lectin pathway and the alternative pathway, and is part of the etiopathogenesis. However, more studies are required to analyse its prognostic value in kidney function, and thus serve as a basis for establishing treatments focusing on complement pathways.
FundingThis work has been funded by the University of Guanajuato, Mexico (CIIC133/2022).
Conflicts of interestThe authors have no conflicts of interest to declare.