Complement 3 (C3) glomerulopathy is diagnosed when C3 dominant glomerulonephritis is seen in kidney biopsy with C3 only deposition without immunoglobulin (Ig), or dominant C3 with up to 1+IgM, or dominant C3 of +2 orders of magnitude of intensity by immunoflourescent (IF) greater than any other immune reactant (using a scale of 0 to 3, including 0, trace, 1+, 2+, 3+).1 Systemic lupus erythematosus (SLE) lead to renal damage through immune deposition such as IgG, IgA, IgM, C3, and C1q, with IgG dominance or codominance in a specific pattern known as full-house.2
A 49 year old male patient applied to our clinic due to high serum creatinine levels noticed at dermatology department during examination for discoid rash. He was well until his skin eruptions have erosen one month ago. Hydroxychloroquine and topical corticosteroid were prescribed to him for cutaneous lupus erythematosus diagnosed by skin biopsy. His blood pressure was 120/80mmHg. Trace pretibial edema and hypopigmented lesions on forearms were detected. Biochemically, his serum creatinine (Cr) level was 1.28mg/dL, estimated glomerular filtration rate (eGFR) Chronic Kidney Disease Epidemiology Collaboration equation (CKD–EPI-cre based): 66mL/min/1.73m2, serum albumin level was 3.6g/dL, proteinuria was 660mg/day, his urine sediment was inactive at admission. Kidneys were ultrasonographically normal in size and echogenicity. Double-checked result of proteinuria level was 1.87g/day, complement 3 (C3) and complement 4 (C4) levels were normal (104mg/dL, normal range=90–180; and 16mg/dL, normal range=10–40 respectively), antinuclear antibody (ANA) was detected positive at 1/1000–1/3200 titration by IFA (immunofluorescence assay), anti-double-stranded deoxyribonucleic acid (anti-dsDNA) antibody level was found as 176.2IU/mL (negative titration=<100IU/mL) by IFA, presence of perinuclear (myeloperoxidase) anti-neutrophil cytoplasmic antibodies (ANCA) was observed by IFA with a serum titration between 1/32 and 1/100 together with positive anti-SS-A, anti-Smith (anti-Sm), anti-histone, and anti-nucleosome antibodies tested by immunoblot analysis.
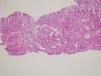
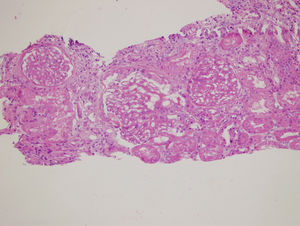
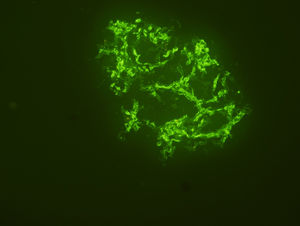
Renal biopsy revealed membranoproliferative glomerulonephritis with diffuse glomerular basal membrane thickening and global mesangial matrix expansion by light microscopy (Fig. 1). Six of the 17 glomeruli were globally sclerotic. No cellular/fibrous crescent and necrosis was noticed. Direct immunofluorescence microscopy displayed granular full-house staining with predominant intense C3 staining (severity degree of +3) (Fig. 2), followed by C1q (mild staining), and IgG (mild staining), in addition to lambda (moderate staining), kappa (mild staining) and fibrin (severe staining). C4d staining showed presence of C4d deposition. Autoantibody test results and findings of skin biopsy made us thought lupus nephritis at first. However kidney biopsy revealed findings associated with both lupus nephritis class IV-G (A/C) and C3 dominant glomerulopathy. The dominant C3 deposition made it necessary to research molecular genetic complement disorders.3,4 In our patient, further examinations in order to enlighten C3 glomerulopathy, yielded homozygous p.His402Tyr mutation due to c.1204C>T change in the complement factor H (CFH) gene and homozygous p.Val306fs mutation due to c.914_915insA insertion in the complement 3 (C3) gene and heterozygous p.Lys565Glu mutation due to c.1693Ag/day. Methylprednisolone and mycophenolate mofetil were given to the patient because he developed hypersensitivity to cyclophosphamide. Proteinuria level decreased to 2.56g/day, serum creatinine level decreased to 1mg/dL, and serum albumin level increased to 3.9g/dL after 1 year of follow-up.
Our patient was diagnosed as SLE by fulfillment of either the 1997 American Collage of Rheumatology (ACR) criteria and by the 2012 Systemic Lupus International Collaborating Clinics (SLICC) criteria.5 The signature of lupus nephritis in renal pathology is polyclonal staining of IgG, IgA, IgM, C3 and C1q with dominant or codominant IgG.2 There was no dominant IgG deposits, even no uniform involvement of IgG and C3 deposits in our case.6 Instead, C3 dominance fulfilled the criterion necessary to diagnose C3 glomerulopathy defined by consensus report of International Society of Nephrology.1 Dysregulation of complement system due to mutations or antibodies lead to C3 glomerulopathy. Only ≅25% of cases diagnosed as C3 glomerulopathy were reported to have genetic mutations in genes of C3 (encoding complement factor 3), CFB (encoding complement factor B), CFH (encoding complement factor H, the regulatory protein of compleman activation), CFI (encoding complement factor I, inactivator of C3b), and CFHR5 (encoding complement factor H-related protein 5, enhancer of complement activation).7 The c.1204c>T; p.His402Tyr variant in the CFH gene has been reported to be highly associated with dense deposit disease and favorable outcomes in age-related macular degeneration.8,9 This variant of CFH put our patient at an increased risk for liability to complement-mediated diseases which emerge in adulthood. The second variant in genetic sequence of complement factor B gene was probably pathogenic for complement mediated disorders like thrombotic microangiopathy as reported previously.10 It remains to be determined whether the third genetical variant in complement 3 gene is capable of causing complement mediated disease. The data about mutation in the complement factor B gene of our patient and its clinical importance for enhanced formation and delay in inactivation of C3bBb convertase needs to be searched.
In conclusion, as far as we know this is the first case showing the togetherness of C3 glomerulopathy and lupus nephritis. After one year of treatment with methylprednisolone and mycophenolate mofetil, renal improvement was achieved. Further studies will enlighten the best therapeutic approach for this new entity in the future.
Authorship contributionsConcept: Kubra Kaynar. Design: Kubra Kaynar, Beyhan Güvercin. Control: Kubra Kaynar, Beyhan Güvercin, Sahile Safarlı, Sevdegül Mungan, Mustafa Şahin. Data Collection: Sahile Safarlı, Sevdegül Mungan, Mustafa Şahin. Literature review: Kubra Kaynar. Writing the manuscript: Kubra Kaynar.
Compliance with ethical standardsAll authors declare that there are no conflicts of interests related to the study and no fund was taken. Informed consent was obtained from patient.