The infection by the BK Polyomavirus (BKV) is an emerging problem in kidney transplants that contributes to a chronic loss of kidney grafts, and in which immunosuppression plays a decisive role. Understanding its risk factors and strictly monitoring urine and serological markers of the infection could mitigate the undesirable effects of this disease. In this review, we investigate the clinical and epidemiological aspects of the BKV infection, as well as go over the available prophylactic and treatment methods currently available for controlling the infection in kidney transplant patients that receive modern immunosuppression.
La infección por el poliomavirus BK (PBK) es un problema emergente en el trasplante renal que contribuye a la pérdida crónica de los injertos renales, y en el que la inmunosupresión desempeña un papel decisivo en su aparición. El conocimiento de los factores de riesgo y la monitorización estrecha de marcadores urinarios y serológicos de la infección pueden mitigar los efectos indeseables de esta infección. En esta revisión se profundiza en los aspectos clínicos y epidemiológicos de la infección por PBK, así como en las medias profilácticas y terapéuticas disponibles para su control en pacientes con trasplante renal que reciben moderna inmunosupresión.
INTRODUCTION
Viruses are pathogens that are especially problematic in transplant recipients, since the issue of viral infections in transplantations reflects a complex equilibrium between the various viral infections that a patient may have throughout his/her lifetime, the antiviral immune response of the recipient, and the level of immunosuppression required for ensuring a functioning graft.
Infection by polyomavirus BK (BKV) is an emergent problem in kidney transplants, and is considered to be the price paid for modern and powerful immunosuppression (IS).
BIOLOGICAL ASPECTS OF THE VIRUS
The polyomavirus, along with the papillomavirus, belongs to the papovavirus family of pathogens. The BK virus (BKV) belongs to the polyomavirus family along with other polyomaviruses that have been detected in humans, such as the JC virus (JCV), the KI virus, the WU virus, the Merkel cell carcinoma virus, and the Simian virus 40 (SV40).
These are small, non-enveloped viruses, with a diameter of 42 nm. The capsid has icosahedral symmetry and houses a double circular chain genome of DNA with over 5000 base pairs, composed of an “early” region that is highly conserved and codes for the “T/t antigen” (TAg), which is implicated in transformation, viral replication, and gene regulation and expression; and a “late” region that codes for the three capsid proteins, known as VP1, VP2, and VP3, and for a protein called “agnoprotein,” a non-coding regulatory region situated between the other two, where the determinants for replication, the TAg union, and transcriptional regulation elements are located.
The polyomavirus possesses adaptation specificity to its host; therefore, its evolution is probably associated with the host species’ evolution, and so the natural infection occurs only in a limited number of closely related species, constituting a marker for establishing the racial differences between humans.
Using gene-sequencing analysis, different genotypes have been established: European, Asian, and African. The rest of the genotypes correspond to recombinations of these three, and although its origin is difficult to establish, the study of this virus could provide a tool for aiding in understanding the evolution of human migrations.
BKV is associated with two complications observed in transplant recipients: BK virus-associated nephropathy (BKVN) in kidney transplants, and haemorrhagic cystitis in bone marrow transplants. In contrast to the BKV, although the JCV resides in the uroepithelium and normally reactivates, it rarely produces nephropathy, but is associated with multifocal leukoencephalopathy and encephalitis. SV40, which comes from simians, was introduced into the human population through vaccines contaminated with polio and adenovirus, and although its presence has been detected in transplanted kidney biopsies, its importance in kidney transplantation is not yet well defined.
EPIDEMIOLOGY AND RISK FACTORS
The primary infection occurs subclinically during the first decade of life, with a seroprevalence of over 80% in the adult population.
The source of infection is exclusively human, no animals have been shown to act as reservoirs, and the transmission route can be faecal-oral, respiratory, transplacental, and through donated tissues. During the viremic phase, the virus infects the tissues, urothelium, lymph tissue, and brain, producing a latent lytic infection.
After the natural viral transmission during infancy, the BKV remains in the urinary tract with intermittent reactivations and low levels of viruria (Vr), 5%–10% in immunocompetent adults.1,2 In immunocompromised individuals, the frequency of BK Vr increases to 20%–60%, and even greater levels of viruria and the appearance of decoy cells in urine are also frequent.3
In kidney transplantation, the prevalence of nephropathies associated with BK virus (BKVN) oscillates between 1% and 10%,4 based more on the immunosuppression treatment and diagnostic methods than due to real epidemiological differences.
In 2004, the treatment of the BKV infection after kidney transplantation was included in the American database as a variable for post-transplant evolution (TBKV); the data were later analysed, resulting in a total of >48 000 transplants, 1474 of which were treated within 24 months. The cumulative incidence of TBKV increased with time, going from 3.45% at 24 months to 6.6% at 60 months after the transplantation.
Graft failure secondary to BKVN occurs at a rate of 50%–100% at 24 months in centres with no screening programs, which highlights the importance of an early diagnosis of the disease.5
Different IS protocols have been identified as risk factors for the development of BKVN, especially the use of triple therapies with anticalcineurinic drugs, mycophenolate mofetil (MMF), and steroids,5,6 but BKVN cases have also been described when using other IS regimens, which indicates that the intensity of IS treatment, and not the specific drug itself, is the risk factor in this case. Other types risk factors also exist, such as patient factors (males >50 years of age, BKV seronegative recipient), graft factors (BKV seropositive donor, HLA incompatibilities, immunological or ischemic injury), and viral factors (latent viral load, capsid serotype, and capacity for replication).7
BKVN HISTOLOGICAL DIAGNOSIS AND PROGRESSION
Decoy cells, viruria, and viremia only indicate viral replication, not nephropathy, but they are key tools for preventing and monitoring the disease.
The only clinical sign of BKVN is the deterioration of kidney function, and when this occurs, it is already too late to intervene, since the renal damage has already been produced.
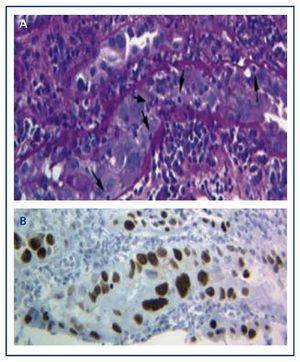
The diagnosis of the disease can only be performed with a graft biopsy in which the typical basophilic nuclear viral inclusions are found in the epithelial cells (tubular, Bowman’s capsule, and/or urothelium), and signs of inflammation with tubulitis (Figure 1A), similar findings to those that appear in acute transplant rejection by T-cells. Only by using the immunohistochemical technique for SV-40 LTAg can we observe a positive nuclear staining and identify the polyomavirus (BK, JC) as that responsible for the inflammation, thus discarding the diagnosis of acute T-cell rejection (Figure 1B) and confirming the diagnosis of BKVN.
BKVN histological lesions are focal and heterogeneous, and so a negative biopsy cannot exclude the diagnosis. As such, this test must be repeated if the viral load in the patient’s blood remains persistently high.
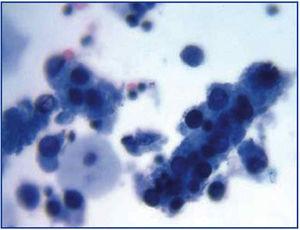
The histological patterns of BKVN2,8,9 are based on the identification and extension of the inflammatory infiltrate and viral infection-associated fibrosis, which allows for three histological patterns to be established (Figure 2).
CLINICAL EVOLUTION AND OPPORTUNITIES FOR EARLY PREVENTION AND DIAGNOSIS
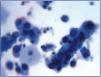
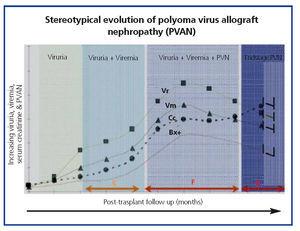
The common clinical evolution of BKVN9 is represented in Figure 3, which shows how the development of the disease is predicted by the appearance of BK viruria (BK Vr), a consequence of viral reactivation and replication in the urinary tract, with the appearance of typical decoy cells (figure 4), which are easy to identify using routine urine cytology tests. However quantification of Vr using PCR techniques is more sensitive than using cytology, and allows for distinguishing between BKV and JCV infections.
When viruria is >105 copies/ml and persists, it is followed weeks or months later by the development of viremia (Vm) at >107 copies/ml and, finally, BKVN. BK Vr is not diagnostic of renal parenchymal damage, but the simultaneous appearance of Vm and Vr is pathognomonic of renal parenchymal damage (BKVN). Maintained, or more typical, increasing Vm is a predictive factor for deteriorating kidney function, and is correlated with the presence and severity of histological lesions. In patients with normal or moderately low kidney function, the probability of finding histological indicators of BKVN is directly proportional to the duration and severity of viremia. Elevated and sustained viremia identifies those patients with uncontrolled viral replication that leads to kidney damage.
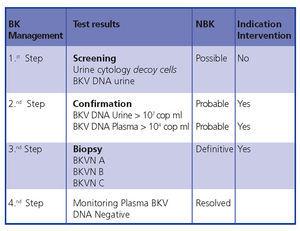
In conclusion, early diagnosis and intervention minimises the damage to the transplant. Figure 5 demonstrates a diagnostic algorithm based on previous publications.4,9
BKVN TREATMENT
The best treatment for BKVN is an early diagnosis of the disease in order to act before renal damage is caused.
For this reason, KDIGO Guides10 suggest using a screening process for all kidney transplant patients by testing monthly Vm levels during the first 3 months (2D) and every three moths until the end of the first year (2D), whenever renal dysfunction is produced with no visible alternative cause (2D), and after treatment for en episode of acute rejection (2D). A reduction in IS is also suggested when Vm is persistently greater than 107 copies/ml (2D).
Regarding the reduction in IS, the first step consists of implementing the standard protocol (not giving CAN or antiproliferative treatments above the levels indicated for the therapeutic range), followed by measuring viremia every 4 weeks, reducing NAb by 15%-20%, reducing MMF and/or MMF suppression by 50%, and/or substituting TAC by CsA or an ISP (Figure 6).11
With regard to antiviral treatments, i.v. immunoglobulins, ciclofovir, leflunomide, and quinolones have been used empirically, and their efficacy is currently difficult to determine because they have not been administered in combination with a reduction in IS and because of the lack of controlled and randomised prospective studies.
Finally, we would like to comment on kidney retransplantation in patients that have lost a graft due to BKVN. The recurrence of the disease in short studies is 12%. The recommendations that must be taken into account in these situations are: 1) inform the patient as to the increased potential risk of recurrence of BKVN; 2) confirm the absence of viral replication (blood and urine PCR when the patient is included on the transplant list and every 6 months thereafter), the patient must receive the transplant with negative PCR results from blood samples, and 3) adapt the IS to the pathology.12-14
KEY POINTS
1. The powerful and modern forms of immunosuppression could be responsible for the increasing prevalence of this infection
2. BK virus infection in immunocompromised patients could affect the function and survival of kidney transplants
3. Early diagnosis by strictly monitoring urine decoy cell count and/or viruria and viremia is crucial for avoiding the negative impacts of this complication
4. No evidence exists of a specific effective treatment for this infection. Only a reduction in immunosuppression treatment can minimise virulence.
Figure 1. Basophilic nuclear viral inclusions in epithelial cells and tubulitis in the BK-virus nephropathy (a) and immunohistochemistry for the antigen SV-40 LTAg (b)
Figure 2. Histological patterns of the BK virus-associated nephropathy
Figure 3. Disperse decoy cells and cellular cylinders containing compacted decoy cells. When they appear, these cylinders are pathognomic of kidney damage
Figure 4. Phases of evolution of the BK virus-associated nephropathy
Figure 5. BKVN diagnostic algorithm
Figure 6. BKVN treatment algorithm