We describe the course of a boy with atypical haemolytic uraemic syndrome (aHUS) with no mutation in the complement system, who presented with two unusual extrarenal complications; osteonecrosis of both femoral heads and cholelithiasis, resulting from a relapse of aHUS caused after suspending eculizumab treatment.
The case is an 8-year-old boy with a neonatal onset of aHUS with severe and multisystemic disease. By administering eculizumab, it was achieved full haematological and renal recovery.1 He continued the treatment with eculizumab from the neonatal period to the age of three years and six months, and the disease was maintained in complete remission.
Exhaustive testing of all the known genes that could cause aHUS was carried out and no mutations were found. Therefore the treatment was discontinued. The genes studied were: ADAMSTS13, ARMS2, C1S, C2, C3, C3ARI, C4BPA, C4BPAP1, C4BPAP2, C4BPB, C5, C5ARI, C6, C7, C8A, C8B, C8G, C9, CD46, CD46, CD55, CD59, CFB, CFD, CFH, CFHR1, CFHR2, CFHR3, CFHR4, CFHR5, CFI, CFP, CLU, CPB2, CR1, CR1L, CR2, CRP, DGKE, F12, F2, F3, FCN1, HTRA1, KDR, MASP1, MASP2, MBL2, NR5A2, PHG, PIGA, PLG, PROC, PROCR, PROS1, PTX3, RP1, RP1L1, SELP, SERPING1, TFP1, THBD, THBS1, VSIG4, VTN, and VWF. No variants (haplotypes) of risk in the MCP gene or the CFH gene were found.
Three months later, after mild cold symptoms, he was admitted for acute kidney failure. Treatment was resumed with eculizumab, and all parameters wee normalized within a few days (Table 1).
Laboratory test data over time.
| Age (years) | 1 | 3 | 3.5 | 3.6 | 4 | 6 | 8 |
| Creatinine, plasma mg/dl | 0.34 | 0.28 | 0.99 | 0.33 | 0.31 | 0.49 | 0.56 |
| Urea, plasma mg/dl | 35 | 36 | 149 | 35 | 41 | 42 | 42 |
| Cystatin mg/l | 0.93 | RELAPSE | 0.89 | 0.85 | |||
| Haematocrit % | 38 | 38 | 26 | 30 | 39 | 40.2 | 40 |
| Platelets 10³/μl | 239,000 | 166,000 | 13,000 | 174,300 | 180,000 | 204,000 | 181,000 |
| LDH U/l | 262 | 303 | 2218 | 342 | 243 | 203 | 215 |
| Prot/Cr in urine | 0.51 | 0.45 | 6.1 | 3.55 | 0.25 | 0.16 | 0.22 |
| Complement C3 (g/l) | 1.16 | 1.12 | 1.18 | 1.37 | 1.02 | 1.1 |
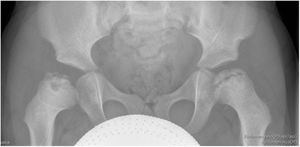
One year after the last relapse, it was detected by chance, synchronic and asymmetric bilateral necrosis of both femoral heads (Fig. 1). Based on the stage of progression at which the lesions were found, the osteonecrosis occurred during the relapse caused after withdrawing the medication. The absence of a history of degenerative coxofemoral joint disease and other secondary causes of osteonecrosis enabled other possible aetiologies to be ruled out.
In the same study, asymptomatic gallstones were detected.
Five years later, the child continues to receive treatment with eculizumab, with good tolerance (Table 1), without presenting evidence of thrombotic microangiopathy (TMA) activity.
DiscussionIn aHUS, the membrane attack complex causes damage to the endothelium, and this triggers TMA.
Although the aHUS lesions predominantly affect the kidney vessels, the diffuse and systemic nature of TMA leads to microvasculature involvement of other organs (brain, heart, intestines, pancreas, lungs, etc.).2
In addition to the triad of haemolytic anaemia, thrombocytopoenia, and acute kidney failure, our patient presented avascular necrosis of both femoral heads, presumably caused by the systemic TMA that occurred during the relapse. Damage to the microvascular endothelium in the terminal circulation of the femoral head would cause tissue hypoperfusion with vascular supply interruption, bone infarction, and ultimately necrosis in both femoral heads.3 Its association with aHUS has not been reported.
Among gastrointestinal complications, some cases of gallstones have been published in the literature several months after an acute episode of typical HUS4; however, this complication is rare in aHUS.
One of the most currently debated topics is the duration of treatment with eculizumab5,6 since the decision to withdraw treatment is not risk-free, especially in patients with a very severe and life-threatening clinical presentation of aHUS at the start. This controversy is accentuated in the paediatric age, since the common events that lead to complement activation (infections, vaccines, etc.) are common in this age group.
No mutations in the complement genes are identified in 30–40% of patients with aHUS. There is evidence that the severity of aHUS and the response to eculizumab is similar in patients with or without an identified genetic risk,6 although maintaining the treatment is more difficult in patients in whom no mutations were found in the complement system.
FundingNo funding was received for this study.