Cardiovascular morbidity and mortality in chronic kidney disease (CKD) patients remain unacceptably high, as evidenced by large epidemiological studies and registries of renal patients from the Spanish autonomous communities and world-wide.1
Both etiology and clinical manifestations have differential aspects compared to the general population. CKD patients have accelerated atheromatosis and ischemic events from early stages of the disease. However, contrary to the general population, the implications of lipid alterations are controversial.
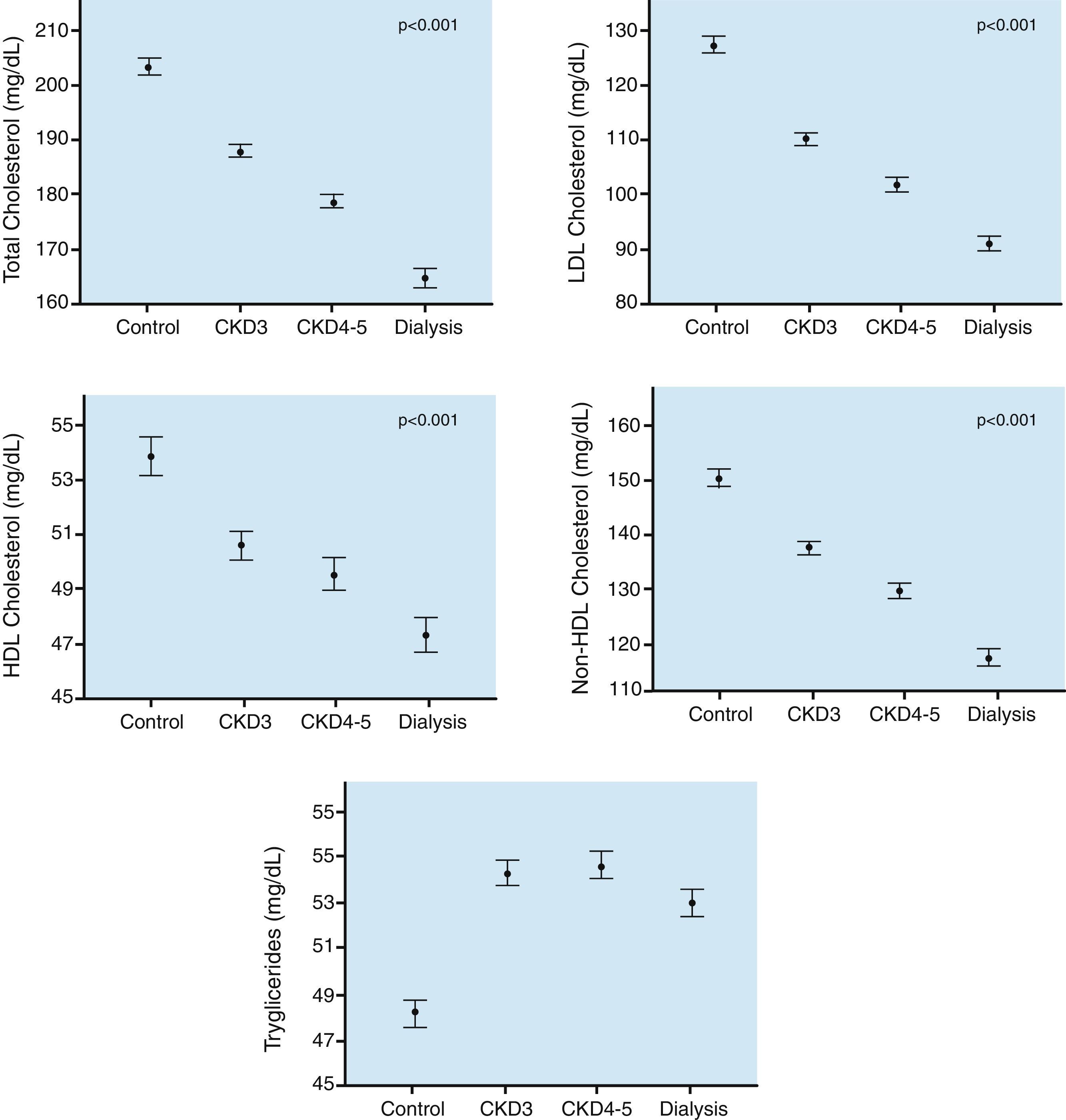
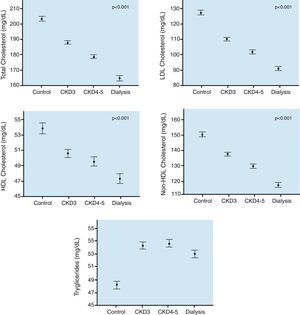
First, the lipids of patients with CKD show a different profile from the dyslipidemia of the general population, and it varies with the severity of renal dysfunction. In CKD, lipid abnormalities are characterized by hypertriglyceridemia, variable levels of LDL-cholesterol and low levels of HDL-cholesterol. In early CKD stages, there are high levels of LDL-cholesterol, but in more advanced stages this parameter is normalized or even reduced.2 The National Observatory of Atherosclerosis in Nephrology (the NEFRONA Study)3,4 observed a progressive decrease of total cholesterol, LDL-cholesterol, HDL-cholesterol and non-HDL-cholesterol that are proportional to the stage of renal disease. (Fig. 1).
Traditional lipid parameters in CKD.
Data obtained from the NEFRONA study.5 Values are expressed as mean±standard error.
Second, renal patients have a higher burden of atheromatosis than the general population, and it increases with the severity of renal dysfunction.5 In addition, they show a more rapid progression of atherosclerosis, particularly in advanced CKD stages.6a Atheromatosis is not only a predictor of vascular events, it is a vascular disease in itself, and as recently stated by the European Society of Atherosclerosis, LDL-cholesterol is an ethiological factor. In fact, high LDL-cholesterol is a condition necessary for the development of a sequence of events leading to the formation of the atherosclerotic plaque, whose growth or rupture is the final trigger of the vascular event itself.6b
Third, studies on therapeutic interventions increased the controversy. Statins, lipid-lowering drugs with highly demonstrated effectiveness in the general population, appeared to be less effective in patients with CKD. The well known studies 4D7 and AURORA8 failed to demonstrate the effectiveness of statins in dialysis patients. In addition, the SHARP study reinforced this idea, although it showed that in non-dialysis CKD patients, statins did reduce vascular risk. Hofwever, this reduction decreased as renal function declines.9 It is important to bear in mind when analysing these results that the above studies have some specific circumstances and limitations. Nonetheless, their results and conclusions have been reproduced many times, and have been strengthened by the publication of multiple meta-analyzes 10–13
In addition, a debate has been generated by the latest American guidelines of vascular risk, which were also supported by the 2013 KDIGO guidelines on the management of dyslipidemia in CKD. In both guidelines, a paradigm change was promulgated in the management of lipid disorders and the prevention of vascular events. They encourage the use of pharmacological treatment based on the patient's risk profile and omitting LDL-cholesterol values. The guidelines are summarized in the following points. First, it is recommended to study the lipid profile of all patients with CKD of any stage. Most do not require follow-up controls. Second, it is recommended to initiate statin or statin / ezetimibe in CKD patients over 50 years of age not on dialysis. Third, in patients under 50 years of age not on dialysis and renal transplants of any age, it is suggested to treat only if they have a history of cardio or cerebrovascular event, diabetes or an estimated 10-year risk of cardiovascular event greater than 10%. In both cases, the indication for treatment is not guided by LDL-cholesterol level, and dislipemia follow-ups are not recommended. Finally, in patients on dialysis, it is not suggested to initiate lipid-lowering treatment.14
These recommendations were received with unequal reactions, especially the one that refers to no need for follow-up lab measurements, an strategy of “shoot and forget”. This is similar to what was suggested months before by the American College of Cardiology and the American Heart Association for the general population.15 This disturbing change was soon rejected by many European scientific societies as well as by many American experts. However, having no intention to make an analysis in detail of these statements, they reflect a gap between physicians who are faced with risk management and vascular prevention.
The latest European guidelines of the European Society of Cardiology and the European Association of Atheromatosis of 2016 classify patients with CKD as subjects with moderate vascular risk (if the estimated glomerular filtration rate (eGFR) is 30–59mL/min/1.73m2) or severe (if the eGFR<30mL/min/1.73m2).16 This classification has therapeutic implications since it sets the target LDL-cholesterol levels in 100 or 70mg/dL, respectively.
All this controversies have generated different ideas and heterogeneity in clinical practice in relation to the diagnosis, treatment and the assessment of the contribution of dislipemia to the cardiovascular morbidity in these patients.
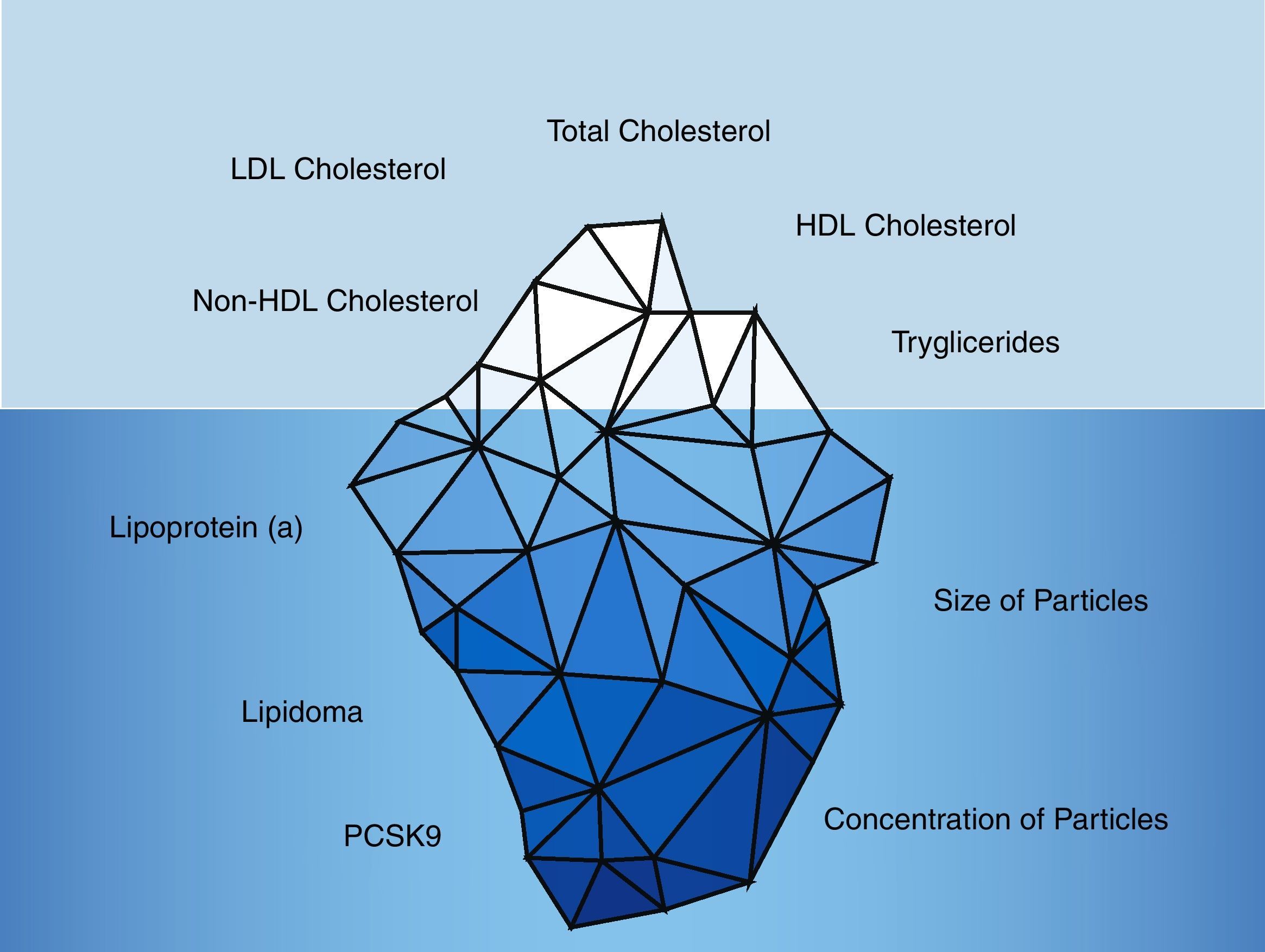
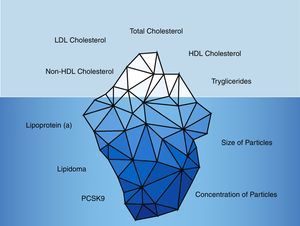
Historically, the lipid parameters measured in clinical practice were: total cholesterol, LDL-cholesterol, HDL-cholesterol and triglycerides. However, in recent years the limited contribution of these parameters in the renal population has become evident (Fig. 2).
Traditional and non-traditional lipid parameters.
The traditional lipid parameters are based on total cholesterol, LDL-cholesterol, HDL-cholesterol, non-HDL-cholesterol and triglycerides. However, non-traditional parameters such as the lipidoma, particle size and number of particles along with lipoprotein(a) and PCSK9 levels would provide more accurate information about the vascular risk of CKD patients.
In CKD patients, the nearly normal or low levels of LDL-cholesterol do not explain the fast progression and elevated atheromatous burden6a,17, and the considerable residual risk.13
Nowadays, there are new approaches to analyze the different subpopulations of lipoproteins; among many options, the most common are: gel electrophoresis, density gradient ultracentrifugation and nuclear magnetic resonance.18 Briefly, each method determines different physicochemical parameters of lipoproteins such as size, electrical charge, cholesterol concentration or the magnetic resonance to assess the lipoprotein subclass distribution.19
Based on the current knowledge, we propose a new approach to the management of lipid abnormalities in CKD patients; the idea is to identify specific therapeutic targets in this population. It is fundamental for clinicians to know what is measured in a routine lipid profile. The parameter “LDL-cholesterol” or “HDL-cholesterol” reflects the concentration of cholesterol transported in molecules called LDL or HDL, respectively. However, cholesterol may be transported in a variable number of LDL or HDL particles, thus the load of cholesterol per particle would not be the same. Therefore, it makes sense, to measure the concentration of LDL and HDL particles (LDL-P and HDL-P); individuals with the same LDL-cholesterol may have different vascular risk if they have different particle concentration. The individual with higher LDL-P has higher cardiovascular risk.20 Conversely, a high HDL-P correlates with a reduced vascular risk.21 The size of the particle is relevant. Multiple studies have shown that small LDL particles are more atherogenic than large ones since they have an increased ability to penetrate the vascular wall.22 Similarly, small HDL particles also correlate with a higher vascular risk due to a decreased anti-atherogenic effect.23 Renal patients have an accumulation of small LDL particles that are associated with a higher rate of cardiovascular events.24,25 Interestingly, they show a decrease in small pro-atherogenic HDL particles.26 Paradoxically, these parameters of considerable clinical relevance are currently not determined in daily clinical practice although their use in research is becoming widespread.
Lipoprotein (a) [Lp (a)] is one parameter easy to obtain from the clinical Lab. It has been demonstrated that the levels of Lp(a) increase as kidney disease progresses27 and decline after kidney transplantation.28 An in vivo study showed a decrease in the clearance of Lp(a) in hemodialysis patients, demonstrating the involvement of the kidney in its elimination.29 Unlike patients on hemodialysis, patients with nephrotic syndrome have an increased hepatic synthesis of Lp(a) resulting in an elevation of plasma levels.30 Lp(a) has a marked proatherogenic effect.31 Lp(a) predicts the development of carotid atheromatous disease32,33 and vascular events in dialysis patients.34 However, the determination of Lp(a) is not widespread, which can be explained by the absence of drugs capable to modify Lp(a) levels.
Historically, available drugs only aimed to modify the concentration of LDL-cholesterol. There were no treatments with a significant effect on other lipid parameters (aside from apheresis). On the other hand, PCSK9 inhibitors offer a new therapeutic approach. They have the ability to reduce of LDL cholesterol levels and the risk of vascular events.35 Moreover, they also reduce the plasma concentration of Lp(a).36 It should be emphasized that these new drugs exert an impact on the size of the particles. They reduce the total concentration of LDL particles and also cause a reduction of large and small LDL particles. Surprisingly, they increase the number of HDL particles, especially the larger ones.37 Unfortunately, in Nephrology, we do not have specific studies that evaluate the impact of these treatments in patients with CKD. A priori, given their beneficial effect on parameters altered in CKD, it is easy to expect positive results. Based on that, the nephrology community need to design clinical trials in such population.
Meanwhile, it is urgent to gain further information about specific lipid abnormalities in renal patients. Besides the quantitative changes described above, CKD is characterized by qualitative changes caused by a highly inflammatory and pro-oxidative state.38 New research tools, such as metabolomics and lipidomics, are useful to investigate lipid abnormalities in CKD patients. There are reasons to believe that during the next coming years, we will witness a resurgence of the field of dyslipidemia in Nephrology, with new perspectives and more effective tools.
Conflict of interestThe authors declare that there is no conflict of interest.
We are grateful to all members of the Vascular and Renal Translational Research Group in the IRBLleida, Spain
Please cite this article as: Bermúdez-López M, Betriu À, Valdivielso JM, Bretones del Pino T, Arroyo D, Fernández E. Más allá de los parámetros lipídicos tradicionales en la enfermedad renal crónica. Nefrologia. 2018;38:109–113.