We report a case of low-grade B-cell lymphoma of mucosa-associated lymphoid tissue (MALT) involving the left kidney and simultaneous onset of a monoclonal gammopathy IgM kappa. No predisposing local inflammatory condition was identified. Following left nephrectomy, the renal specimen showed the centrocyte like cells and lymphoid cells in the lymphoepithelial lesions were positive for CD20 and CD79α. The neoplastic cells expressed monotypic cytoplasmic IgM kappa. The demonstration of bone marrow cells of B-lineage expressing the same monoclonal protein as the tumor suggested bone marrow involvement, even in the absence of identical morphology. Despite chemotherapy and rituximab treatment, clinical follow-up showed right kidney extension with high-grade transformation, and finally systemic dissemination. This case illustrates that the kidney is among the sites that may be involved by MALT B-cell lymphomas in a primary or secondary fashion, and the need for expanded investigation of the possible dissemination. We review the literature on this unusual extranodal lymphoma.
Se presenta un caso de linfoma de células B de bajo grado del tejido linfoide asociado a mucosas (MALT), afectando al riñón izquierdo, y comienzo simultáneo de una gammapatía monoclonal IgM kappa. En este paciente no pudo identificarse ningún proceso inflamatorio predisponente local. Tras la nefrectomía izquierda, el espécimen renal mostró células centrocito-like y células linfoides en las lesiones linfoepiteliales que fueron positivas para CD20 y CD79 alfa. Las células neoplásicas expresaron IgM kappa monotípica citoplásmica. La demostración de células de estirpe B de la médula ósea expresando la misma proteína monoclonal que el tumor sugirió la afectación de la médula ósea incluso en ausencia de idéntica morfología. A pesar del tratamiento con quimioterapia y rituximab, el seguimiento clínico demostró extensión al riñón derecho, con transformación a linfoma de alto grado y, finalmente, diseminación sistémica. Este caso ilustra que el riñón se encuentra entre las localizaciones que pueden verse afectadas por los linfomas de células B de tipo MALT, de forma primaria o secundaria, y explica la necesidad de extender la investigación para detectar su posible diseminación. Se revisó la literatura sobre este infrecuente linfoma extranodal.
Introduction
Isaacson and Wright [1] initially defined malignant lymphoma of the mucosa-associated lymphoid tissue (MALT) in the gastrointestinal tract, and subsequently in the thyroid, lung, and salivary gland, as a neoplastic proliferation of centrocyte-like cells, with or without lymphoplasmacytic cells, usually accompanied by lymphoepithelial lesions and benign-appearing germinal centers [2]. More recently, B-cell lymphomas of MALT arising of a variety of sites have been described, including the breast, orbit, conjunctiva, skin, gallbladder, cervix, larynx, and trachea [2-4]. Urogenital tract is among the various sites involved in MALT lymphoma [3-4]. However, B-cell lymphomas of MALT type involving the kidney are relatively rare [4-20]. The present paper describes the clinical presentation, pathological features and disease course of a patient with MALT B-cell lymphoma involving the kidney and a serum M-component of IgM kappa.
Case report
A 77-year-old man with a 30 years history of hypertension was referred to our hospital for evaluation of chronic renal failure. Four years before the patient had been diagnosed of Barrett's esophagus and duodenal ulcer with pyloric stenosis by upper gastrointestinal endoscopy. Esophagus biopsies showed esophagitis with koilocytosis. The biopsy urease test was negative but he received specific treatment to H. pylori eradication. At that time laboratory data revealed hematocrit 40 %, hemoglobin 12.5 g/dl, white blood cells 5380/ml with normal differential count, and an erythrocyte sedimentation rate of 20 mm/h (normal 15-30 mm/h). Total serum proteins were 5 g/dl and albumin 3.1 g/dl. Urinalysis was normal and serum creatinine ranged from 1.2 to 1.4 mg/dl. He continued receiving treatment with omeprazole, and sixteen months later a barium study of the upper gastrointestinal tract showed normal findings.
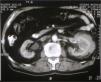
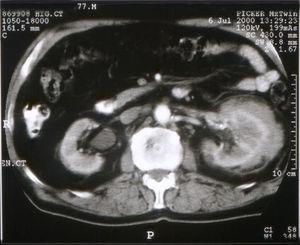
At admission physical examination was unremarkable. Laboratory data showed hematocrit 34.5 %, hemoglobin 11 g/dl, white blood cells 5950/ml with normal differential count, platelets 161000/ml, and an erythrocyte sedimentation rate of 100 mm/h. Blood coagulation was normal. Urinalysis revealed normal sediment and proteinuria 1.2 g/24 h. Serum urea was 70 mg/dl, serum creatinine 2.2 mg/dl, and creatinine clearance 45 ml/min. The total serum proteins were 9.6 g/dl, albumin 4.1 g/dl, and gammaglobulin 3.5 g/dl (with a monoclonal peak). The serum immunoglobulin levels measured by nephelometry were IgM 4640 mg/dl (normal 38-231 mg/dl), IgG 858 mg/dl (normal 650-1700 mg/dl), and IgA 168 mg/dl (normal 103-568 mg/dl), kappa 641 mg/dl (normal 170-370 mg/dl), lambda 106 mg/dl (normal 90-210 mg/dl). Monoclonal IgM kappa was detected in the serum by immunofixation. Serum cryoglobulin was negative and Bence-Jones proteinuria was positive. Other laboratory data showed C reactive protein 4.9 mg/dl, beta 2-microglobulin 4.9 mg/l, lactate dehydrogenase 286 U/l, serum calcium 9.9 mg/dl, and serum phosphate 3.2 mg/dl. EBV and HCV were negative. Abdominal ultrasound examination showed a solid mass in the left kidney. A skeletal roentgenogram and a bone scan revealed no abnormalities. A thoracoabdominal CT revealed a 7 cm mass located in the midportion of the left kidney (Fig. 1). There was no evidence of lymphadenopathy. A bone marrow aspirate showed a normocellular patter and revealed 12.5 % atypical plasma cells. Bone marrow flow cytometry showed 3 % polyclonal T lymphocytes and 1 % polyclonal B lymphocytes. The plasma cells were CD38++, CD138-, CD19+, CD45+, CD56-, CD117-, and monoclonal kappa.
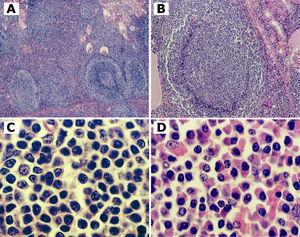
On September 2000, a left nephrectomy was performed. The kidney weighed 240 g (including the adrenal gland), measured 20 x 12 x 8 cm, and contained an 8 x 8 x 2 cm well-circumscribed mass involving the subcortical mid-portion of the kidney. Histologically, there was a lymphoid infiltrate extending diffusely through the pericapsular region and involving the renal cortex. Kidney architecture was effaced by the infiltrate within which occasional residual tubules and glomeruli were evident (Fig 2 A). In some areas, the infiltrate was diffuse between the reactive lymphoid follicles. Many reactive germinal centers were colonized by neoplastic cell with a residual mantle zone preserved (Fig 2 B). In other areas, the neoplasm was nodular, composed of reactive lymphoid follicles surrounded by pale zones of small lymphoid cells with pale or clear cytoplasm and slightly irregular nuclear contours (monocytoid and centrocyte-like cells) (Fig 2 C). In some areas, a prominent plasma cell and plasmacytoid lymphocytes component with occasional intranuclear inclusions (Dutcher bodies) and Russell bodies was observed (Fig 2 D). The neoplastic cells expressed monotypic cytoplasmic IgM kappa by immunoperoxidase. The neoplastic cells also reacted with the pan-B-cell antibody L26 (CD20, CD79a), coexpression of CD43 and stain of atypical lymphocytes with bcl-2 consistent with a low-grade B-cell lymphoma of MALT. Lymphoepithelial lesions were occasionally observed with cytokeratin immunostain. In the areas of the kidney not involved by lymphoma, there were histologic signs of nephrosclerosis.
Two months after nephrectomy the serum creatinine was 3 mg/dl and the erythrocyte sedimentation rate remained elevated to 141 mm/h. The total serum proteins were 8.4 g/dl, albumin 4.1 g/dl, and gammaglobulin 2.4 g/dl with a monoclonal peak. The serum immunoglobulin levels were IgM 2970 mg/ml, IgG 865 mg/ml, and IgA 161 mg/ml. The patient was well and symptoms free, and he refused any invasive exploration. He received treatment with chlorambucil 5 mg/week and despite of this the monoclonal peak (IgM kappa) persisted and the serum IgM level remained elevated. The patient was followed by monthly routine blood examinations. On April 2003, an abdominal CT revealed a mass located in his solitary right kidney. A fine needle aspiration biopsy of the kidney revealed a B clonal kappa, CD19+, CD10+ and CD20+ cell infiltration. A high-grade MALT lymphoma transformation was considered and the patient was treated simultaneously with CHOP (cyclophosphamide, doxorubicin, vincristine and prednisone) chemotherapy and a course of rituximab. On May 26, a second cycle of CHOP was initiated but he developed severe neutropenia and multiple complications, including sepsis and acute renal failure necessitating hemodialysis. On October 2003, dissemination of the lymphoma occurred and the patient died with multi-organ failure.
Discussion
Extranodal B-cell lymphomas of MALT arise at extranodal sites, are usually associated with chronic inflammation as a result of an infection or autoimmune disorder, and share histologic and immunophenotypic features. At these anatomic sites, MALT lymphomas are commonly associated with inflammatory conditions that predispose to lymphomagenesis. This is best established in the stomach where MALT lymphomas are commonly associated with H. pylori infection [1]. The absence of lymphoid tissue in the normal renal parenchyma and the inability to rule out absolutely the presence of microscopic foci of tumor elsewhere, has led to controversy about the existence of primary renal lymphoma as a distinct disease. However, it has been suggested that lymphoma may arise from the renal hilum or from foci of inflammation that attract lymphocytes to the area, such as chronic pyelonephritis [8, 20]. Other related factors contributing to the genesis of renal MALT lymphoma were Sjögren's syndrome [4], IgA nephropathy [7], membranoproliferative glomerulonephritis [12], Epstein-Barr virus [10], actinomycosis [18], sarcoidosis [15], systemic lupus erythematosus [17], and gastric H. pylori infection [9, 18]. The presence of concomitant renal cell carcinoma [18], transitional cell carcinoma [18] or colonic adenocarcinoma [18] no appears to be a contributing factor.
To our knowledge, only 30 unequivocal cases, non including the one reported here, of B-cell lymphomas of MALT involving the kidney have been reported in the English literature [4-20] (Table I). Except a recent report by Garcia et al. [18] of a series of 10 cases the majority were case reports. It is difficult in these patients to tell in which organ the lymphoma originated. Thus, whereas in 5 cases the primary tumor was localized in the salivary glands, the orbit or the gastrointestinal tract and the kidney lesion represented dissemination [6, 7, 8, 18], in other 2 cases the tumor was simultaneously diagnosed in the kidney and the parotid gland [4, 16], and in other 3 cases MALT lymphoma involving the kidney was associated to enlarged lymph nodes [10, 17, 18]. In all these cases secondary involvement of the kidney cannot be excluded. Only in 18 cases the tumor could have been originated in the kidney [5, 9, 11-15, 18-20].
MALT lymphomas are usually confined to the sites of origin when diagnosed and are slow to disseminate. If disseminated, have a tendency to involve other mucosal organs. This explains the prolonged clinical course and the efficacy of surgical excision with no further therapy in some patients [9, 11, 13-15, 18-20]. Thus, in 8 cases the neoplasm involving the kidney did not recur or disseminate following nephrectomy [9, 11, 13-15, 18-20]. In other case there was a remission following nephrectomy and irradiation [5]. In one case with definitive resolution of the lymphoma following to chlorambucil therapy there was disappearance of serum M-component and improvement in symptomatology [7]. In other case with renal actinomycosis there was a remission following to antibiotics [18]. In other case there was a partial remission at 15 months following nephrectomy and chlorambucil administration [4]. In some cases the tumor had indolent behavior and very slow clinical course. In one case the lymphoma progressed very slowly and the patient remained alive without any treatment for 5 years [8]. Nine patients were alive with no evidence of disease from 9 to 53 months [18, 19]. Three patients, including our case, died of disease from 3 to 13 years after initial diagnosis [6, 10], 2 of who presented high-grade transformation and dissemination of the lymphoma.
The lymphoma cells express monotypic surface immunoglobulins or, to a lesser extent, cytoplasmic immunoglobulins, usually IgM [15]. On the other hand, monoclonal gammopathy is a common phenomenon in patients with MALT lymphoma, most probably due to paraprotein production by the clonal lymphoplasmacytic cells [21-23]. In a study the monoclonal gammopathy corresponded with the light chains detected on biopsy by immunohistochemical on the majority of cases [22]. In 18 of 31 cases of B-cell MALT-type lymphoma of the kidney (including the one reported here) monotypic cytoplasmic immunoglobulins kappa or lambda were present. However, only 3 cases of monoclonal gammopathy IgM, one with the light chain of the lambda type and 2 with kappa, have been reported so far [7, 12].
The pathogenesis of B-cell lymphoma in our case is unknown, as there was no chronic inflammation identified in the kidney. The case reported here is singular as it showed the morphologic, immunologic, and phenotypic features of a B-cell MALT-type lymphoma of the kidney occurring in a patient with a monoclonal gammopathy IgM kappa. Thus, in our case, monoclonal gammopathy corresponded with the light chain detected on kidney by immunohistochemical. On the other hand, the demonstration of bone marrow cells of B-lineage expressing the same monoclonal protein as the tumor suggested bone marrow involvement, even in the absence of identical morphology. In the literature we identified only another case of MALT lymphoma involving the kidney and bone marrow [18]. In addition, at 2 months of the nephrectomy the persistence of the serum M-component was suggestive of incomplete remission. Despite chemotherapy and rituximab treatment, monoclonal gammopathy IgM kappa persisted. Clinical follow-up showed right kidney extension with high-grade transformation, and finally systemic dissemination. Therefore, the presence of monoclonal gammopathy was associated with more advanced disease, with bone marrow involvement and high-grade transformation [23]. Although MALT lymphoma seems to be a relatively benign disease in most patients, the clinical course of our patient clearly demonstrated the malignant potential and the importance of prompt and aggressive treatment. As occurred in our patient, adverse prognosis factors may include presence of monoclonal gammopathy, bone marrow involvement, high tumor burden, high-grade transformation, or dissemination of disease [24].
In summary, this case illustrates that the kidney is among the sites that may be involved by MALT B-cell lymphomas in a primary or secondary fashion, and the need for expanded investigation of the possible relation with systemic involvement. These lymphomas, like other extranodal MALT-type lymphomas, have indolent behaviour and slow clinical course as demonstrated by the available literature. However, in some cases high-grade transformation and/or dissemination of disease occur. The treatment choice should be patient-tailored, taking into account the site, the stage and the clinical characteristics of the individual patient.
Figure 1. CT showing tumoral growth through the renal cortex of the left kidney.
Figure 2.
10428_18107_6542_en_10428_table_1.doc
Table 1. Summary of clinical and pathologic features of B-cell lymphoma of MALT involving the kidney