To the Editor:
Autosomal dominant polycystic kidney disease (ADPKD) is the most common hereditary cause of terminal chronic renal failure (CRF), with an incidence of 1 in 500-1000. This disease is produced by mutations to the genes PKD1 (16p13.3, 85%) or PKD2 (4q22.1, 15%). In ADPKD, the growth of renal cysts produces a progressive increase in renal volume and destruction of the parenchyma, leading to terminal CRF at approximately 50-60 years of age (in PKD1 mutations). Although ADPKD is bilateral, renal involvement may be asynchronous and asymmetrical,2 and in PKD2 mutations, terminal CRF may be delayed by as many as 20 years with respect to PKD1. On the other hand, congenital anomalies of the kidney and urinary tract (CAKUT) are the most common cause of CRF in children, constituting half of all cases.3-6 Although many cases of CAKUT are caused by unique genetic defects, mutations have been identified in only a few genes. These unique mutations can produce a wide phenotypic spectrum of CAKUT, which ranges from vesicoureteral reflux to renal agenesis.5-7 Unilateral renal agenesis is a congenital anomaly that occurs in 1 in 3000 births,3 for which several candidate genes exist. The coexistence of ADPKD and unilateral renal agenesis occurs in approximately 1 in 1 500 000-3 000 000 individuals. As such, the coincidence of both anomalies in a single individual is very rare, with only 7 cases reported.8-12
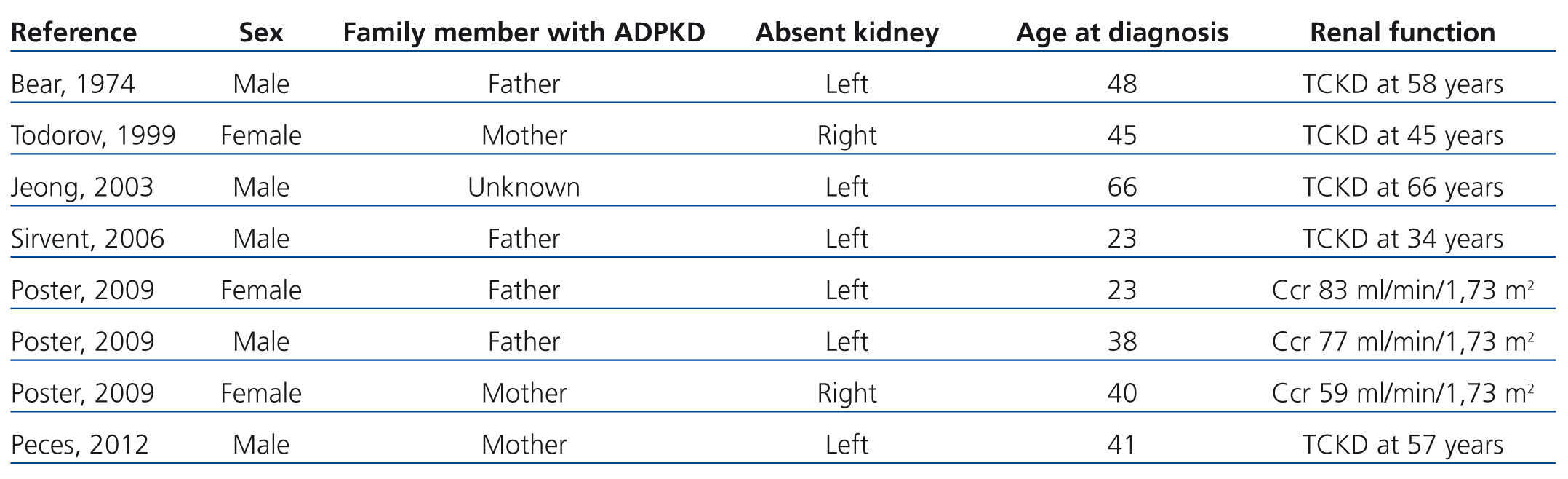
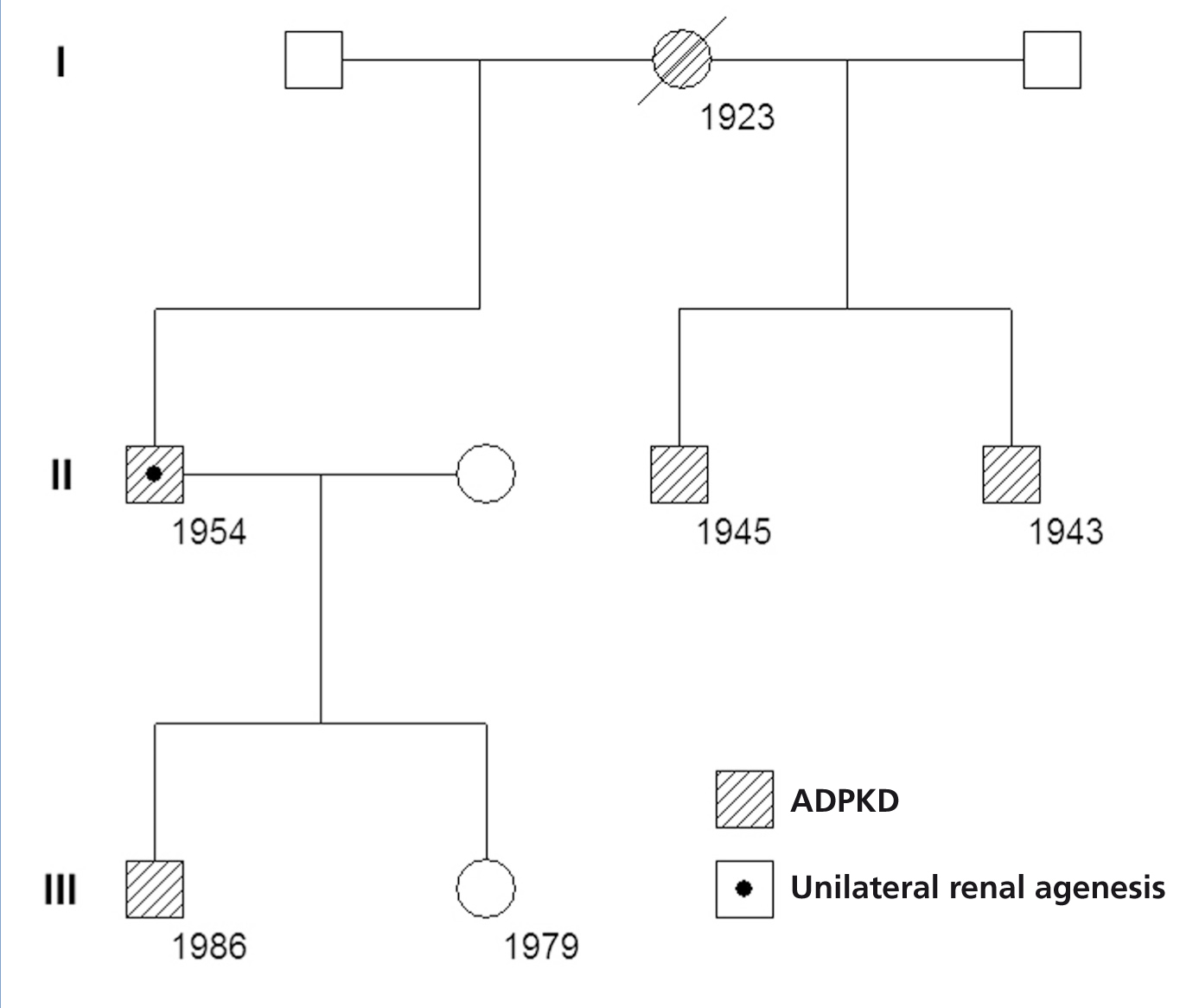
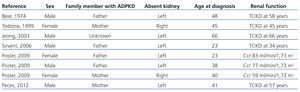
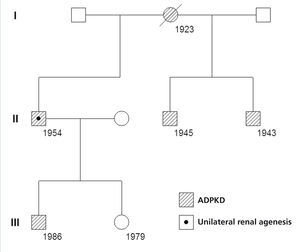
In a cohort of 205 patients with ADPKD, we identified a new case of ADPKD with unilateral renal agenesis, which is the 8th such case reported in the world and only the 2nd in Spain. Our patient was a 57-year old male who was diagnosed with right ADPKD and left renal agenesis by ultrasound at age 41. At this moment, the patient’s serum creatinine (Crs) value was 1.2mg/dl. The right ADPKD and left renal agenesis were confirmed by isotopic renogram and magnetic resonance angiography (MRA). The MRA revealed hepatic cysts, a large right polycystic kidney, and absent renal tissue on the left side (Figure 1). Sixteen years later, sCr was 5.9mg/dl and creatinine clearance was 12ml/min/1.73m2 . In the last 6 years, the patient had suffered a mean annual loss in glomerular filtration rate of 7.6ml/min/1.73m2, requiring peritoneal dialysis starting at age 57. In the patient’s family background, the mother, two siblings, and one child all had ADPKD (Figure 2). The family genetic analysis ruled out the possibility of unilateral renal agenesis in other family members.
Renal agenesis is produced by a lack of interaction between the ureteric bud and the metanephric mesenchyme, leading to the absence of ureter and kidney. Induction of the ureteric bud from the nephric duct is mediated by glia cell-derived neurotrophic factor (GDNF), which is secreted by the metanephric mesenchyme and interacts with the tyrosine kinase c-Ret receptor expressed in the ureteric bud in order to induce branching of the nephric duct.13 Several candidate genes have been isolated: BMP4, RET, GDNF,FREM2 (FRAS1-related extracellular matrix protein 2), and FRAS1 (Fraser syndrome 1).13-16 FRAS1 and FREM2 are responsible for maintaining the integrity of several renal epithelial structures, and are also implicated in the initiation of metanephric kidneys. The gene FRAS1 codes for the protein FRAS1, which contains repeats of the primary domain of sulphate chondroitin proteoglycan, whose function is to maintain the integrity of the epithelial cells. The protein FRAS1 was detected in several tissues under development. In the metanephros, FRAS1 was detected in the extracellular matrix, covering the basal surface of the ureter during growth and the basal membrane of the collecting tubule. In individuals with Fraser syndrome, all FRAS1 mutations reported were mixed heterozygotes or homozygotes, indicating a recessive genetic trait. In contrast, in non-syndromic unilateral renal agenesis, unique FRAS1 heterozygotic mutations have been observed.16 On the other hand, unilateral renal agenesis is associated with congenital anomalies of the reproductive system and urinary tract (cysts in the seminal vesicles).17 Approximately 40%-50% of children with renal agenesis also have other urogenital defects, such as vesicoureteral reflux, ectopic kidney, pelvicalyceal dilatation, ureteral duplication, neurogenic bladder, cryptorchidism, and structural defects of the vagina or uterus. In addition, 15% of these children have cardiovascular defects.18
The importance of the association between ADPKD and contralateral renal agenesis is due to the possible causal relationship between these two processes and due to the influence that a single polycystic kidney may have on the early development of CRF. Of the 8 cases of ADPKD and unilateral renal agenesis (including our case) recorded in the medical literature, only 2 underwent genetic analyses to differentiate between PKD1 and PKD2,12 and each case was associated with a different mutation of the PKD1 gene. When unilateral renal agenesis occurs as an isolated anomaly, it is asymptomatic, tends to be discovered incidentally during routine ultrasound tests, and normally produces a compensatory hypertrophy of the contralateral kidney. In the case of association with ADPKD, this compensatory hypertrophy may not occur, thus accelerating the development of CRF in the single polycystic kidney. In this manner, unilateral ADPKD would represent a model for analysing the consequences of reducing the number of available nephrons in ADPKD. The cases of ADPKD in a single kidney do not provide sufficient information regarding kidney volume or rates of progression,8-12 and several already had advanced CRF (Table 1). In addition, these cases of ADPKD in a single kidney cannot be compared to those of patients with two polycystic kidneys. The 2 patients described by Poster12 who had unilateral ADPKD had higher renal volumes and rates of volume increase than the mean values recorded in a control group. With the exception of the case reported by Sirvent,11 which involved severe uncontrolled hypertension, the 7 cases reported of unilateral ADPKD did not experience accelerated rates of disease progression. Our patient progressed towards terminal CRF, with an annual loss in renal function of 7.6ml/min/1.73m2 during the last 6 years. As such, the available data indicate that unilateral ADPKD kidneys do not appear to retain the capacity to compensate for the reduction in the number of available nephrons. Unilateral renal agenesis also occurs most commonly on the left side of the body,19 since in 6 out of the 8 cases reported to date of ADPKD and contralateral renal agenesis, the absent kidney was the left kidney (75% of cases). No clear explanation has been put forth for this predominance, highlighting our lack of understanding of embryogenesis and nephrogenesis in particular.
To conclude, this case illustrates the coincidence of two hereditary renal diseases: one autosomal dominant, ADPKD, and the other recessive, contralateral renal agenesis. Although ADPKD and unilateral renal agenesis can be due to alterations to 2 different and independent genes, we cannot rule out the possibility of a causal relationship between these two anomalies.20 More cases will need to be analysed in order to come to a conclusion in this regard.
Acknowledgements
This study was financed in part by the Carlos III Institute of Health, the Ministry of Science and Innovation (EC08/00236) and the programme for intensifying research activities (IdiPAZ and Agencia Lain-Entralgo/CM) to R.P.
Conflicts of interest
The authors state that they have no potential conflicts of interest related to the content of this article.
Table 1. Patients with autosomal dominant polycystic kidney disease and contralateral renal agenesis as reported in the medical literature
Figure 1. Coronal magnetic resonance image demonstrating a large right polycystic kidney and absent left kidney. The liver also has multiple cysts
Figure 2. Genealogical tree of the family with autosomal dominant polycystic kidney disease and unilateral renal agenesis. Numbers indicate date of birth