Patients with advanced chronic kidney disease (CKD) are at greatest risk of hyperkalemia (HK). The relationship between HK and negative outcomes (mortality or progression of renal insufficiency) in non-dialysis dependent CKD patients is controversial.
AimsTo determine the incidence, prevalence, and factors related with HK in a cohort of CKD patients, and its relationship with mortality, hospitalization rate, CKD progression, and dialysis initiation.
Material and methodsA retrospective, observational study in an incident cohort of adult patients with stage 4 or 5 CKD not on dialysis. Inclusion criteria were: having at least three consecutive estimated glomerular filtration rate (eGFR) measurements in a follow-up period >3 months. Decline in renal function was estimated as the slope of the individual linear regression line of eGFR over follow-up time. HK was defined as serum K levels ≥5.5meq/l. Associations of HK with outcomes were adjusted for major confounding variables in the multivariate analysis.
ResultsThe study group consisted of 1079 patients (574 males, mean age: 65±14 years) with mean baseline eGFR 14.8±4.5ml/min/1.73m2. Mean follow-up time was 15 months with a median of 7 serum sample determinations per patient. HK was observed at baseline in 26% of patients; in at least one serum sample during the individual follow-up period in 68%; or chronically (>50% of samples) in 33% of patients. By multivariate logistic regression, the best determinants of chronic HK were: male sex (OR=1.529; 95% CI [1.154–2.025], p=.003), serum bicarbonate (OR=0.863 [0.829–0.900], p<.0001), diuretic treatment (OR=0.743 [0.556–0.992], p=.044), and angiotensin converting enzyme inhibitor and/or angiotensin receptor blockers (OR=4.412 [2.915–6.678], p<.0001). Patients whose serum K levels were in the upper quartile showed a significantly faster CKD progression (−4.05±5.22 vs. −2.69±5.61ml/min/1.73m2/year, p<.0001), and more frequent dialysis initiation (63% vs. 57%, p=.115), though lower mortality (9% vs. 17%, p=.003) and hospitalization rates (2.68±5.94 vs. 3.16±6.77 days per year, p=.301) than the other study patients. However, in the multivariate analysis, average serum K levels were not independently associated with the clinical outcomes investigated.
ConclusionHK is a common biochemical finding in non-dialysis dependent CKD patients, mainly associated with prescribed medication. However, HK was not independently associated with major negative clinical outcomes.
Los pacientes con enfermedad renal crónica (ERC) tienen un alto riesgo de desarrollo de hiperkaliemia (HK). La relación entre HK y una mala evolución (mortalidad o progresión de la insuficiencia renal) en la ERC avanzada es controvertida.
ObjetivosDeterminar la incidencia, prevalencia, y factores relacionados con la HK en una cohorte de pacientes con ERC, y su relación con la mortalidad, tasa de hospitalización, progresión de la ERC, y necesidad de inicio de diálisis.
Material y métodosEstudio retrospectivo de observación en una cohorte de pacientes adultos con ERC estadio 4–5. Los criterios de inclusión fueron: tener al menos 3 medidas consecutivas de filtrado glomerular (FG) durante un periodo superior a 3 meses. HK se definió como un K sérico ≥ 5,5mmol/l. La asociación entre HK y las variables de evolución fue ajustada a los principales factores de confusión mediante análisis mutivariantes.
ResultadosSe incluyeron 1079 pacientes (574 hombres, edad media: 65 ± 14 años) con un FG basal 14,8 ± 4,5ml/min/1,73m2. El tiempo medio de seguimiento fue de 15 meses y se determinaron una mediana de 7 muestras por paciente. Basalmente un 26% de pacientes tenía HK, un 68% en al menos una muestra durante el periodo individual de seguimiento, y un 33% de forma crónica (HK > 50% del seguimiento individual). Mediante regresión logística multivariable los mejores determinantes de la HK fueron: sexo masculino (OR = 1,529; IC 95% [1,154-2,025], p = 0,003), bicarbonato sérico (OR = 0,863, [0,829-0,900], p < 0,0001), tratamiento diurético (OR = 0,743, [0,556-0,992], p = 0,044), y tratamiento con inhibidores del sistema renina-angiotensina (OR = 4,412, [2,915-6,678], p < 0,0001). Estos pacientes con HK mostraron una progresión de la ERC significativamente más acelerada (−4,05 ± 5,22 vs. −2,69 ± 5,61ml/min/1,73 m2/año, p < 0,0001), e inicio más frecuente de diálisis (63% vs. 57%, p = 0,115), pero menos mortalidad (9% vs. 17%, p = 0,003), y tasa de hospitalización (2,68 ± 5,94 vs. 3,16 ± 6,77 días/año, p = 0,301) que el resto de los pacientes estudiados. Sin embargo en el análisis multivariante, HK no se asoció de forma independiente con ninguna de las variables de evolución investigadas.
ConclusiónHK es un hallazgo bioquímico muy frecuente en la ERC avanzada, que se asocia con algunos medicamentos de uso habitual. Sin embargo, HK no se asocia de forma independiente con ninguna de las variables de mala evolución clínica estudiadas.
Hyperkalemia (HK) is a very common electrolyte disorder in renal failure.1–6
Due to the frequent association between HK and serious, even lethal, adverse effects, there is an almost atavistic fear of this ionic disorder in clinical practice, which usually imposes the need to correct it without taking into account other circumstances and evaluations.7
Patients with chronic kidney disease (CKD) usually have mild-moderate and persistent elevations in serum potassium levels. The association between HK and the development of serious adverse effects or poor clinical outcome in CKD is controversial.4–17 In addition, the administration of drugs that are currently used to treat HK18,19 or the discontinuation of medications that favor HK7,8,20–25 may cause other complications which add confusion to the relationship between HK and clinical evolution.
We have had a long experience in diminishing the importance of HK in advanced CKD; the strategy have been to avoid severe dietary restrictions, no prescription of drugs for the specific treatment of HK such as cation exchange resins or fludrocortisone, which are usually poorly tolerated and with potential serious adverse effects, and maintaining the prescription of drugs such as the renin-angiotensin system inhibitors. This has allowed us to gather a cohort of numerous patients follow for a period long enough to analyze the adverse effects and evolution associated with this electrolyte alteration, taking into consideration some important confounding factors.
Thus, the objectives of the present study were: to determine the incidence, prevalence, and factors related to HK in a cohort of patients with CKD, and its relationship with mortality, hospitalization rate, progression of CKD, and the need to initiate regular dialysis.
Material and methodsThis is a retrospective longitudinal observational study in a cohort of adult patients diagnosed with CKD stages 4–5 not on dialysis, followed in the advanced CKD (ACKD) outpatient clinic from January 2000 to December 2016. The selection criteria were: being followed in the ERCA clinic during a period greater than 3 months with at least 3 measurements of renal function, serum potassium and other biochemical parameters of interest.
All patients were referred to the ACKD clinic for progressive deterioration of renal function. Demographic, clinical, and prescribed medication data were obtained from medical records, physical examination and anamnesis. Comorbidity was assessed at the time of inclusion using the Davies index,26 and patients were categorized into three groups: no comorbidity, mild-moderate, or severe.
All biochemical samples and analytes were obtained and measured in the same central laboratory (Clinical Analysis Service of Infanta Cristina Hospital) by conventional methods (Advia Chemistry Autoanalyzer, Siemens Healthcare Diagnostics, New York, USA), in fresh samples (not stored), and both creatinine calibrations and traceability were performed in accordance with the recommendations of international standards NKDEP.27 Glomerular filtration rate was estimated by the abbreviated formula MDRD.28
The measurement of K concentration was performed in serum, not plasma. To detect possible interference and errors in the measurement of serum K, a systematic review was performed using a hemolytic index, and all samples with a value considered as significant interference (emollitic h index>40) were discarded. In case of pathological increase in the number of blood elements (polycythemia or thrombocytosis), plasma levels of K were also measured, and the existence of pseudohyperkalaemia was investigated by calculating the difference between serum K and plasma K concentration.
The patients were followed regularly with visits to de clinic scheduled every 30–90 days. To determine the rate of progression of renal function deterioration, a linear regression was performed in each patient between the estimated glomerular filtration rate in each control and the time elapsed since the first appointment, with an accuracy of days. The resulting slope of this linear equation was expressed in±ml/min/1.73m2/year, with the negative or positive values of this parameter having the meanings of progression of renal insufficiency or recovery of baseline renal function, respectively.
Electrolyte abnormalities in all patients included in this study were treated uniformly with therapeutic measures that consisted of: no restriction of fresh fruits or vegetables except for severe HK (serum K≥6.5mmol/l), attempted correction of the metabolic acidosis with oral sodium bicarbonate, no prescription in any case of cation exchange resins or fludrocortisone, immediate restriction or suspension of digoxin, non-steroidal anti-inflammatory drugs and mineralocorticoid receptor antagonists (spironolactone, eplerenone), no suspension of conversion enzyme inhibitors of angiotensin (ACEI) or antagonist angiotensin receptors (ARA) or diuretic prescription for the sole reason of HK.
Study design and statistical analysisRetrospective longitudinal observational study in a cohort of patients with advanced CKD. In each patient, all serum potassium (K) measurements obtained during follow-up were recorded. HK was considered a concentration of K≥5.5mmol/l. It was defined as chronic HK when the total percentage of concentrations of K≥5.5mmol/l observed during each individual follow-up was equal to or greater than 50%.
The outcome variables analyzed in this study were: death from any cause before the start of renal replacement therapy, onset of dialysis, hospital admission rate (as a subrogated index of morbidity), and rate of progression of CKD (slope of the relationship glomerular filtration/time).
The determinants of HK were analyzed by multivariate logistic regression, including as dependent variables the following clinical and biochemical parameters of relevance: age, sex, body mass index, active smoker, diabetes mellitus, systolic and diastolic blood pressure, glomerular filtration, proteinuria, serum bicarbonate, diuretic treatment, SRAA inhibitors, beta blockers, calcium channel blockers.
A univariate analysis was performed to evaluate the association between quartiles of the average of K serum concentration during the follow-up time with mortality and onset of dialysis by means of Kaplan–Meier curves. The predictive value of the HK on the progression of CKD, time until the onset of dialysis or mortality were analyzed by multivariate linear regression and Cox proportional risk regression, respectively, together with the variables shown as determinants of HK, adding the comorbidity index. Patients were censored at the time of death, loss of follow-up, onset of dialysis, or end of follow-up (May 1, 2017), whichever occurred first.
The choice of independent variables in multivariate models was done automatically through the process of conditional backwards progressive elimination.
In the analysis of the determinants of the rate of progression of CKD, patients were excluded if, even with an advanced deterioration of renal function, were on specific treatment for glomerulonephritis or vasculitis.
For the descriptive comparison of the continuous variables and depending on their characteristics, parametric or non-parametric tests were used, and chi-square test was used for categorical variables.
Descriptive statistical data are presented as mean and standard deviation, or as median and interquartile ranges for continuous variables, and as percentages for categorical variables. A p<0.05 was considered statistically significant, and all p values shown are bilateral. Statistical analyzes were performed with the IBM SPSS Statistics 24.0 software (IBM Corp. Armonk, USA).
ResultsDuring the study period, a total of 1580 incident patients were treated in the ACKD clinic. 1079 patients (68% of the total patients) were included in the study. The causes of exclusion from the study were not having completed at least 3 months of follow-up due to the need of initiation of regular dialysis (patients that had a late referal to our clinic) in 463 cases, early mortality in 16 patients, recovery of renal function in 16 patients and loss of Follow-up in 6 patients.
The demographic, clinical and biochemical characteristics of the patients included in the study are detailed in Table 1.
Characteristics of the total number of patients included in the study and differences according to quartiles of average serum K levels.
| Variable | Total | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | p |
|---|---|---|---|---|---|---|
| (K≤4.8mmol/l) | (K 4.9–5.2mmol/l) | (K 5.3–5.5mmol/l) | (K≥5.6mmol/l) | |||
| Number of patients | 1079 | 278 | 265 | 268 | 268 | |
| Age, years | 65 (14) | 67 (13) | 66 (14) | 64 (14) | 63 (15) | 0.004 |
| Gender, % men | 53 | Fifty | 49 | 53 | 60 | 0.052 |
| Body mass index, kg/m2 | 29.5 (5.8) | 29.2 (5.9) | 29.9 (5.7) | 29.7 (6.3) | 29.3 (5.5) | 0.524 |
| Comorbidity index: absent/mild-moderate/severe, % | 40/49/11 | 35/51/14 | 37/52/11 | 44/47/9 | 42/46/12 | 0.161 |
| Diabetics, % | 36 | 35 | 37 | 36 | 36 | 0.989 |
| Polycystic kidney disease, % | 8 | 9 | 13 | 5 | 8 | 0.002 |
| Active smoker, % | 17 | 16 | 18 | 16 | 18 | 0.815 |
| Systolic blood pressure, mmHg | 158 (27) | 156 (29) | 159 (25) | 157 (27) | 161 (27) | 0.085 |
| Diastolic blood pressure, mmHg | 87 (14) | 87 (14) | 86 (14) | 87 (15) | 88 (15) | 0.370 |
| Initial glomerular filtration rate, ml/min/1.73m2 | 14.8 (4.5) | 15.7 (4.9) | 14.8 (4.8) | 14, 6 (4.2) | 14.3 (3.9) | 0.002 |
| Initial serum potassium, mmol/l | 5.06 (0.67) | 4.4 (0.5) | 4.9 (0.5) | 5.2 (0.5) | 5.7 (0.6) | <0.0001 |
| Average serum potassium, mmol/l | 5.17 (0.57) | 4.4 (0.3) | 5.0 (0.1) | 5.4 (0.1) | 5.9 (0.3) | <0.0001 |
| Number of determinations/patienta | 7 (5 –12) | 7 (5–12) | 8 (5–12) | 8 (5–12) | 7 (4–11) | 0.149 |
| Determinations showing hyperkalemia (%)a | 25 (0–60) | 0 | 12 (0–20) | 41 (28–50) | 80 (70–100) | <0.0001 |
| Initial serum bicarbonate, mmol/l | 21.7 (3.7) | 23.1 (3.7) | 22.2 (3.8) | 21.3 (3.2) | 20.2 (3.4) | <0.0001 |
| Initial Phosphatemia, mg/dl | 4.6 (1.6) | 4.5 (0.9) | 4.5 (0.9) | 4.6 (1.0) | 4.9 (2.7) | 0.031 |
| Initial serum calcium, mg/dl | 9.2 (0.8) | 9.3 (0.8) | 9.3 (0.9) | 9.2 (0.8) | 9.1 (0.7) | 0.023 |
| Serum albumin, g/dl | 3.9 (1.2) | 4.1 (2.2) | 3.9 (0.4) | 3.9 (0.4) | 3.9 (0.4) | 0.688 |
| Proteinuria, g/g creates tinine | 2.08 (2.36) | 2.19 (2.72) | 2.09 (2.55) | 1.91 (2.23) | 2.12 (1.82) | 0.564 |
| Diuretics,% | 64 | 69 | 64 | 66 | 58 | 0.06 |
| RAS inhibitorsb, % | 75 | 57 | 73 | 85 | 90 | <0.0001 |
| Double blockade of RAS,% | 8 | 6 | 8 | 8 | 9 | 0.561 |
| Beta blockers, % | 25 | 32 | 25 | 24 | Twenty-one | 0.026 |
| Calcium antagonists, % | 48 | 51 | Four. Five | 47 | 47 | 0.503 |
| Statins,% | 51 | 46 | 49 | 51 | 57 | 0.065 |
| EEAc, % | 60 | 60 | 61 | 58 | 62 | 0.732 |
A total of 9990 serum K measurements (median: 7 determinations per patient) were collected, with an individual (median) follow-up time of 15.4 months (IQ ranges: 8.2–27.3 months).
The prevalence of HK in the first baseline determination was 26% (283 patients), HK in at least in one determination throughout the follow-up was present in 68% (732 patients), and chronic HK was observed in 33% (356 patients).
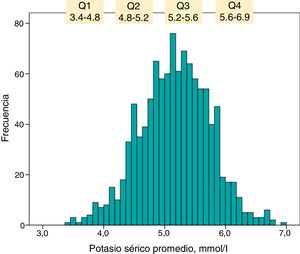
The frequency of distribution of the serum K values averaged at each individual follow-up is represented in the histogram shown in Fig. 1. The upper quartile was formed by patients who maintained an average serum K≥5.6mmol/l throughout the follow-up.
The number of patients with serum K≥6mmol/l was 100, of which 34 had serum K levels≥6.5mmol/l.
The clinical and biochemical characteristics of the patients according to the quartiles of average serum K are shown in Table 1.
Patients who presented HK were characterized by being more often male, younger, with a slightly reduced basal renal function, more metabolic acidosis, treated with renin-angiotensine system inhibitors (iSRA) in 90% of cases (Table 1).
By multivariate logistic regression, the main determinants of HK were: male sex, treatment with diuretics and RASi, and the serum bicarbonate concentration at baseline (Table 2).
Multivariate logistic regression modela on the determinants of hyperkalemia.
| Variable | Odss ratio | 95% CI odds ratio | p |
|---|---|---|---|
| Gender, man=1 | 1.529 | 1.154–2.025 | 0.003 |
| Diuretics, (0,1) | 0.743 | 0.556–0.992 | 0.044 |
| Renin-angiotensin system inhibitors (ACEI/ARA), (0,1) | 4.412 | 2.915–6.678 | <0.0001 |
| Serum bicarbonate, mmol/l | 0.863 | 0.829–0.900 | <0.0001 |
| Constant | 2.700 |
Automatic conditional backwards variable selection.
Outside the best predictive equation: age, comorbidity index, body mass index, systolic blood pressure, diastolic blood pressure, serum phosphorus, basal glomerular filtration rate, proteinuria, diabetes, beta blockers, calcium channel antagonists, double renin -angiotensin system blockers, Erythropoiesis stimulants agents, statin.
Table 3 shows the values of variables in patients according to the quartiles of average serum K.
Variables result in the total group and according to quartiles of average serum potassium.
| Variable | Total | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | p |
|---|---|---|---|---|---|---|
| (K≤4.8mmol/l) | (K 4.9–5.2mmol/l) | (K 5.3–5.5mmol/l) | (K≥5.6mmol/l) | |||
| Slope glomerular filtration regression/time, (ml/min/1.73m2/yeara) | −3.37 (4.68) | −3.44 (4.97) | −2.71 (4.41) | −3.29 (3.98) | −4.02 (5.20) | 0.015 |
| Initiation of dialysis, % | 59 | 54 | 57 | 61 | 63 | 0.139 |
| Death before dialysis onset, % | 15 | 2. 3 | 14 | 13 | 9 | <0.0001 |
| Hospital admission rate, days/year | 3.04 (6.57) | 3.66 (7.19) | 3.08 (6.87) | 2.73 (6.18) | 2.69 (5.95) | 0.277 |
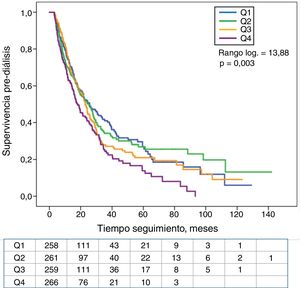
Patients in the upper quartile of serum K showed a more rapid progression of CKD, and a higher frequency of onset of dialysis than in the rest of the patients although this difference was not statistically significant. However, both the percentage of deaths in pre-dialysis stage and the hospital admission rate were higher in the lower quartile of serum K.
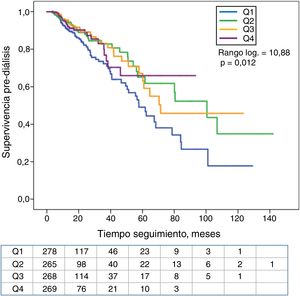
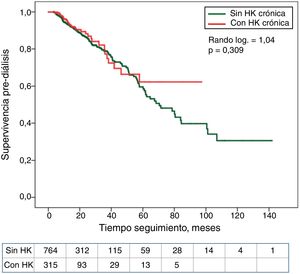
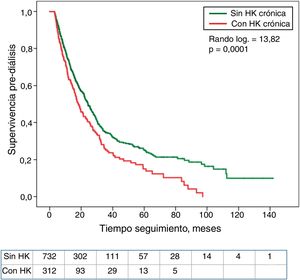
The association between the quartiles of serum K or the diagnosis of HK with the period of time until death or onset of dialysis is shown in Kaplan–Meier curves (Fig. 2). Patients in the lower quartile of serum K showed the worst survival. In contrast, no significant differences were observed in mortality of patients with or without HK (Fig. 3).
Patients in the upper quartile of serum K showed less survival without dialysis than the rest of patients (Fig. 4). Those with HK also needed dialysis significantly earlier than the rest of the patients (Fig. 5).
The association of HK with the rate of progression of CKD was analyzed with multivariate logistic regression, adjusting this result for confounding variables. Table 4 shows the determinants of the best equation of association with the rate of decline of the glomerular filtration rate, among which were neither the serum K concentrations (continuous variable) nor the HK (discrete variable). These two variables were not independently associated with risk ratio (“hazard ratio”) of dialysis initiation, analyzed by Cox regression, or with a higher annual income rate as detailed in Table 5.
Multivariate linear regression on the progression of CKD (slope glomerular filtered ratio/time). Better predictive model and specific value of hyperkaliemia (separate from the best equation).
| Variable | Coefficient B (IC 9 5%) | Beta | p |
|---|---|---|---|
| Age, years | 0.049 (0.030; 0.068) | 0.147 | <0.0001 |
| Body mass index, kg/m2 | 0.091 (0.046; 0.136) | 0.113 | <0.0001 |
| Systolic blood pressure, mmHg | −0.222 (−0.322; −0.122) | −0.128 | <0.0001 |
| Initial glomerular filtration, ml/min/1.73m2 | −0.090 (−0.15 0; −0.030) | −0.085 | 0.003 |
| Initial serum bicarbonate, meq/l | 0.111 (0.039; 0.183) | 0.087 | 0.003 |
| Proteinuria, g/creatinine g | −0.702 (−0.817; −0.586) | −0.344 | 0.000 |
| Double blockade renin-angiotensin system, (0,1) | −1.367 (−2.338; −0.396) | −0.079 | 0.006 |
| Beta block before, (0,1) | −0.608 (−1.205; −0.012) | −0.057 | 0.046 |
| Constant | −5.122 (−7.618; −2.625) | ||
| Hyperkalemia, (0,1) | −0.460 (−1.299; 0.379) | −0.045 | 0.282 |
| Average serum K, mmol/l | −0.103 (−0.968; 0.762) | −0.013 | 0.815 |
Variables outside the best prediction equation: sex, comorbidity index, diabetes, smoker, diastolic blood pressure, diuretics, renin-angiotensin system inhibitors (monotherapy), calcium channel antagonists, average serum K and HK.
Multivariate linear regression on rates of annual hospital admissions (days per year). Better predictive model and specific value of hyperkalemia.
| Variable | Coefficient B (95% CI) | Beta | p |
|---|---|---|---|
| Age, years | 0.048 (0.019; 0.076) | 0.101 | 0.001 |
| Comorbidity index, (0,1,2) | 1.681 (1.069; 2.293) | 0.167 | <0.0001 |
| Proteinuria, g/g creatinine | 0.530 (0.371; 0.689) | 0.190 | <0.0001 |
| Diuretics, (0,1) | 1.091 (0.287; 1.896) | 0.080 | 0.008 |
| Renin-angiotensin system inhibitors, (0,1) | −1.268 (−2.142; −0.394) | −0.083 | 0.004 |
| Beta blockers, (0,1) | 1.100 (0.219; 1.980) | 0.073 | 0.014 |
| Constant | −2.382 (−4.379; −0.385) | ||
| Hyperkalemia, (0,1) | 0.109 (−1.117; 1.334) | 0.008 | 0.862 |
| Average serum K, mmol/l | −0.192 (−1.446; 1.062) | −0.017 | 0.764 |
Variables outside the best prediction equation: sex, diabetes, smoker, body mass index, systolic and diastolic blood pressure, initial glomerular filtration rate, serum bicarbonate, calcium channel antagonists, average serum K and HK.
Although in the univariable Cox regression analysis the lowest average serum K concentrations were significantly associated with higher mortality (Table 6), the multivariable adjustment ruled out a significant association of serum K (both hypo- and hyperkalemia) with mortality.
Cox proportional risk regression on mortality during the pre-dialysis period. Better predictive model and specific value of hyperkaliemia (separate from the best equation).
| Variable | Hazard ratioa | 95% CI RHa | p |
|---|---|---|---|
| Age, years | 1.078 | 1.055–1.101 | <0.0001 |
| Comorbidity, (0,1, 2)b | 2.106 | 1.650–2.687 | <0.0001 |
| Smoker, (0,1) | 2.095 | 1.339–3.280 | 0.001 |
| Systolic blood pressure, cmHg | 1.092 | 1.017–1.173 | 0.016 |
| Diastolic blood pressure, cmHg | 0.847 | 0.732–0.981 | 0.026 |
| Proteinuria, g/g creatinine | 1.115 | 1.068–1.165 | <0.0001 |
| Renin-angiotensin system inhibitors (ACEI or ARA), (0,1) | 0.634 | 0.454–0.8 86 | 0.008 |
| Average serum K, mmol/l | 0.636 | 0.416–0.971 | 0.036 |
| Hyperkalemia, (0,1) | 1.375 | 0.780–2.425 | 0.270 |
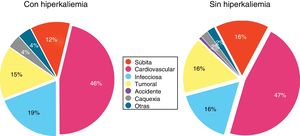
Fig. 6 shows the causes of death in patients with or without HK; no significant differences were observed.
DiscussionThe results of this study in a cohort of patients with advanced CKD show that there is a high incidence and prevalence of HK, not only sporadically but also maintained over time. However, this electrolyte abnormality was not significantly and independently associated with a poor clinical outcome: death before the onset of dialysis, hospital admissions, onset of dialysis, or rate of progression of CKD.
The results of this study, performed in ACKD patients in real every day clinical conditions, show that the best determinants of a maintained HK are being male, the prescription of diuretics and inhibitors of the renin-angiotensin system (RASi), and the severity of metabolic acidosis.
The association between HK and male gender in CKD has been observed in other studies,14,23 although a conclusive explanation for this association is lacking. It has been reported a higher intake of K in men.29 It is also possible that the endogenous production of K by greater cell-mass destruction (muscle) could influence this observation.30
Non-distal diuretics increase the fractional excretion of K and therefore the finding of an association between the prescription of these drugs and a lower incidence of HK is consistent with this explanation.31 On the contrary, in the present study the prescription of RASi was the most important factor in the development of HK, and this is in accordance with most of the observations published so far.1,2,7,16,17,20,24 These findings confirm the importance that pharmacological interference can have with adaptive mechanisms to control K levels in CKD.
The association between metabolic acidosis and HK is well known,16,31,32 and the pathophysiological relationship is mutual,31,32 that is, acidosis promote the increase in serum K levels, and HK may also alter the mechanisms of renal acidification. In addition, the use of RASi could affect both the tubular excretion of K and the acidification mechanisms.33
Due to the association between the use of RASi and HK, very frequently these medications are discontinued,7,8,20–25 and patients are often treated with the addition of diuretics or intestinal K-quelating medications such as cation exchange resins. These practices that pursue the normalization of K levels are not exempt from adverse effects, which have always a factor in the clinical evolution and complications of patients presenting with HK.
The association between HK and adverse effects on CKD is controversial.4–17 The reasons why in our study we did not find a negative effect of this electrolyte alteration in the evolution of the patients could be: (1) the definition of the HK was not based on an isolated determination. (2) Drugs that could have influenced evolution (digoxin, anti-inflammatories, resins, fludro cortisone, diuretics, RASi suspension, etc.) were avoided as part of the treatment and prevention of HK. (3) The correction of metabolic acidosis was a therapeutic priority in all patients. (4) The statistical analysis was adjusted to important confounding variables. (5) The recruitment and inclusion of patients, as well as the diagnosis of HK were not made during a period of time of unstable clinical situations that could cause abrupt elevations or changes in serum K concentration such as decompensations of a heart failure, infections, drug intoxication, severe metabolic acidosis, etc. that could add confusion to the clinical evolution.
Unlike HK, the lower levels of serum K were associated with a worse evolution, and this finding coincides with those observed by many other authors.1,4,8,9,1,14,15,34,35 However, the hypokalemia in CKD, is an alteration that reflects a high comorbidity burden; thus, when the main confounding variables are introduced in evolutionary analyzes, this electrolyte alteration also loses predictive importance as observed in the results of the present study.
The first serum sample of K (baseline) was not included as a variable in the prediction analysis in the present study. Although it could be argued that a single increase in serum K could produce deleterious effects, the inclusion characteristics of the patients in this study require to be alive during the initial 3 moth period, making very unlikely a causal relationship between the initial K and subsequent evolution. Nevertheless, the baseline serum K levels correlated strongly with the obtained as an average throughout the follow-up (R2=0.58; p<0.0001).
Although the HK does not seem to be associated with important variables that predict outcomes in our CKD patients.14–17 HK itself could have other adverse effects such as those associated with neurological disorders (muscle weakness, cognitive abnormalities, polyneuropathies, etc.)36; these are abnormalities that would be worth continuing to analyze and evaluate their possible reversibility after exhaustive control of serum K concentration.
The present study has limitations. Due to the retrospective design, firm causal relationships cannot be established. It was performed in a single center with certain specific criteria in the prevention and treatment of CKD patients, therefore the results may not be generalizable. All patients were Caucasian, therefore it was not possible to determine the existence of differences in tolerance to HK according to racial characteristics, as some researchers have pointed out.16,35
There may be a question about a selection bias due to the minimum follow-up criteria of 3 months. Thus, those patients who for some reason might be more tolerant to HK would have been included, while those not included could have suffered the fatal consequences of HK. However, as detailed in the results, the reason whereby the majority of patients did not complete the initial 3 month of follow up was because they needed to start regular dialysis (these were late referral patients); early mortality before completion of the first 3 month period was very low (3.4%), which is similar to the overall mortality of the patients included in the study.
In conclusion, HK is a very frequent alteration in advanced CKD, mainly related to the use of RASi drugs and metabolic acidosis. However, the HK is not independently and significantly associated with any of the studied variables of poor clinical evolution.
Conflict of interestsThe authors declare no conflict of interest.
Please cite this article as: Caravaca-Fontán F, Valladares J, Díaz-Campillejo R, Barroso S, Luna E, Caravaca F. Asociación entre hiperkaliemia y evolución clínica en la enfermedad renal crónica avanzada. Nefrología. 2019;39:513–522.