Antecedentes: Una de las complicaciones más graves de la cirugía reparatoria de aneurisma aórtico abdominal (AAA) es el fracaso renal agudo (FRA). Incluso un pequeño ascenso de creatinina sérica se asocia a un aumento de la mortalidad. El objetivo de este estudio ha sido valorar la dinámica del FRA después de cirugía electiva de AAA utilizando nuevos marcadores. Métodos: En el estudio se incluyeron 22 pacientes con AAA. Medimos la proteína hepática transportadora de ácidos grasos (u-L-FABP) y la proteína cardíaca transportadora de ácidos grasos (u-h-FABP) en orina, antes, durante y dentro de los tres días siguientes a la cirugía. Resultados: Se observó una brusca y significativa elevación de ambas FABP en orina, normalizada a creatinina en orina; el nivel de u-L-FABP alcanzó su pico dos horas después de quitar la abrazadera aórtica {137,79 (38,57-451,79) frente a 9,99 (6,82-12,42) ng/mg del valor basal p < 0,05; los valores son medianos (cuartil inferior-superior)}. El pico de la u-H-FABP se notó 72 horas después de quitar la abrazadera aórtica {16,462 (4,182-37,595) frente a 0,141 (0,014-0,927) ng/mg del valor basal}. El nivel de creatinina sérica no cambió de manera significativa durante el período de estudio. Conclusiones: El aumento significativo de ambas u-L-FABP y u-H-FABP después de cirugía de AAA indica la lesión tubular renal distal y proximal en la población estudiada. Nuestros resultados sugieren que después de una cirugía de AAA el túbulo distal puede ser más afectado que el proximal. Las u-FABP podrían servir como biomarcadores sensitivos de la lesión tubular renal y permitir detectar la fase más precoz de FRA.
Background: One of the most severe complications of repair surgery for abdominal aortic aneurysms (AAA) is acute kidney injury (AKI). Even small rises in serum creatinine are associated with increased mortality. The aim of this study was to assess the dynamics of AKI after elective AAA surgery using novel markers. Methods: The study group consisted of 22 patients with AAA. We measured urinary liver- (u-L-FABP) and heart-type fatty acid-binding proteins (u-H-FABP) before, during and within 3 days after surgery. Results: We found an abrupt and significant elevation of both urine FABPs normalized to urinary creatinine; u-L-FABP reached its peak value 2 hours after aortic clamp release {137.79 (38.57-451.79) vs. 9.94 (6.82-12.42) ng/mg baseline value, p<0.05; values are medians (lower-upper quartile)}. The peak value of u-H-FABP was reported 72 hours after aortic clamp release {16.462 (4.182-37.595) vs. 0.141 (0.014-0.927) ng/mg baseline value, p<0.05}. The serum creatinine level did not changed significantly during the investigation period. Conclusions: The significant rise of both u-L-FABP and u-H-FABP after AAA surgery indicates renal proximal and distal tubule injury in this population. Our results suggest that, after AAA surgery, the distal tubules could be more affected than the proximal ones. u-FABPs could serve as sensitive biomarkers of kidney tubular injury and may allow to detect the very early phases of AKI.
INTRODUCTION
In the past years, due to the application of new imaging techniques, the number of newly recognized aortic aneurysms has increased considerably. However, the results of surgical treatment are negatively affected by complications among which acute kidney injury (AKI) is regarded as one of the most significant.1,2 Clinical observations have revealed that even minor rises in serum creatinine could have an unfavourable impact on mortality. AKI could result in the necessity of renal replacement therapy, the development of chronic kidney disease or increased mortality.3,4 Up to now, a lot of attention has been directed to meticulous investigation of AKI in critically ill patients, after cardiac surgery or following the use of iodinated contrast mediums.5,6 Despite the fact that the AKI is a well known complication of AAA operations1 less attention was paid to detailed analysis of pathophysiology of this phenomenon. The problem of AKI after AAA surgery is of significant importance because it concerns older people with other comorbidities and very often with already compromised renal function. Moreover, appropriate markers enabling researchers to scrutinize early phases and further dynamics of AKI, which, additionally, outperform serum creatinine, were found only recently.7,8 Among these markers are Liver and Heart Type of Fatty Acid Binding Proteins (L-FABP and H-FABP respectively).9
The family of fatty acid binding proteins (FABPs) comprises nine members. They play an important role in intracellular long-chain fatty acid transport and could have protective effect against cell injury. FABPs are expressed in different tissues with various intensity. Only two FABPs occur in the kidney, ie., H-FABP and L-FABP. Of particular interest is that L-FABP is expressed specifically in proximal tubular cells and H-FABP in distal ones. Therefore, the application of these two cognate markers allows the assessment and simultaneous comparison of the injury to proximal and distal segments of the renal tubule.9-11 While L-FABP has already been used as an efficient AKI marker, H-FABP, up to our current knowledge, has been scarcely used in this context. Nevertheless, a combination of two closely akin markers of the two nephron segments has already been efficiently employed in the assessment of progression of idiopathic membranous nephropathy.12
The aim of this study was to assess the dynamics and severity of kidney injury after AAA surgery using these novel, promising indicators. Furthermore, the selective differential assessment of injury of proximal and distal segment of renal tubules was of our particular interest. We decided to scrutinize early signs of kidney injury during AAA surgery sequentially at different time points with regard to aorta cross-clamping. We would like to analyze the overall dynamics of kidney damage after AAA surgery as indicated by these new markers in typical clinical settings.
MATERIAL AND METHODS
Study population
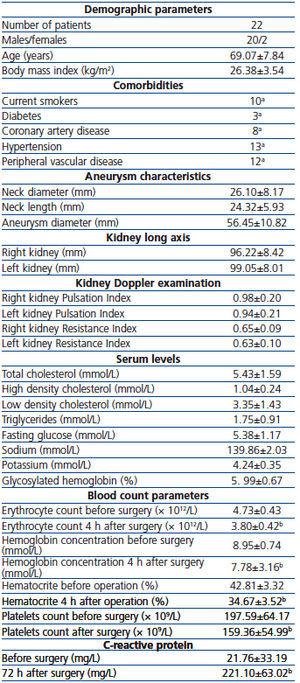
The study comprised 22 consecutive patients (Table 1) admitted to the Department of Vascular Surgery for elective AAA surgery, who signed informed consent and did not meet the exclusion criteria including: (1) the use of aminoglycoside antibiotics within one month before surgery, (2) treatment with cyclosporine A, (3) neoplastic disease, (4) another surgical procedure during one month prior to enrollment, (5) stroke during the preceding two months, (6) myocardial infarction during the preceding three months, (7) essential psychiatric, metabolic, neurological, blood or major internal organs disorders, (8) incident of acute renal failure (ARF) or renal replacement therapy during the preceding six months, (9) an ongoing acute inflammatory response, (10) urinary tract obstruction. The research was approved by the Bioethics Committee of the Medical University of Silesia. Patients characteristics, comorbidities and basic laboratory data are shown in Table 1. Before the enrollment, 7 patients were treated with statins, 11 with ACE-I, one with AT1-bloker, 4 with nonsteroid anti-inflammatory drugs, 12 with β-blockers, 3 with Ca-channel blockers, 4 with nitrates, 2 with diuretics, but none with spironolactone and pentoxyphylline. No patients received nephrotoxic drugs two weeks before and within the study period.
AAA surgery
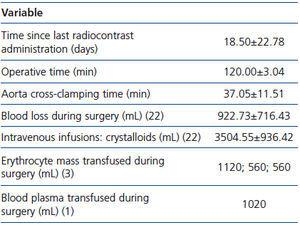
Between days 2 and 108 before surgery the subjects underwent contrast-enhanced computed tomographic angiography. After infra-renal cross-clamping, the aortic aneurysm was excised and the aorta was reconstructed using a PTFE graft. Surgery was performed under general anaesthesia; the details are given in Table 2.
Design
The study protocol was as follows. All admitted patients underwent AAA and kidney ultrasound as well as renal artery Doppler examination. Blood samples were obtained from an upper extremity vein (B-before) on the day before surgery (day - 1). The successive blood samples were drawn from an upper extremity vein: (B4h) 4 hours (day 0), (B24h) 24 hours (day 1), (B48h) 48 hours (day 2), (B72h) 72 hours (day 3), (B96h) 96 hours (day 4) and (B120h) 120 hours (day 5) following the moment of aortic clamp removal. Urine samples were taken (U-before) before operation (day - 1), and then from the urinary catheter (U-surgery) just before removal of aortic clamp, (U2h) 2 hours, (U4h) 4 hours, (U12h) 12 hours (U-surgery – U12h: day 0), (U24h) 24 hours (day 1), (U48h) 48 hours (day 2), and (U72h) 72 hours (day 3) after the removal of aortic clamp. The duration of surgery and aortic clamping, total volume of intravenous infusions and blood loss during surgery were all considered. Complete blood count (samples B-before and B4h) and C-reactive protein CRP (samples B-before and B72h) were assessed. Creatinine concentrations were determined in B-before, B24h, B48h, B72h, B96h, and B120h samples. L-FABP, H-FABP and creatinine concentrations were determined in all urine samples (i.e. U-before – U72h). Estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease Study equation (MDRD) or, alternatively, by CKD-EPI Study formula.13
Laboratory methods
Immediately after clotting blood samples were centrifuged for 15min at 3000g (rotation 4000min-1); frozen serum and urine were stored at -80˚C. Serum and urine creatinine concentrations were determined by the Jaffe colorimetric method. Urine L-FABP and H-FABP were assessed using commercially available enzyme-linked immunosorbent assays (Human L-FABP ELISA Kit and Human H-FABP ELISA Kit Hycult-Biotech, The Netherlands) according to the manufacturer’s instructions. Other laboratory parameters were determined using routine tests.
Statistical analyses
The data were analyzed using Statistica 8.0. computer software. All variables were tested for normality of distribution by means of the Kolmogorov-Smirnov test. Statistical analysis was carried out using non parametric the Kruskal-Wallis/Mann-Whitney U for independent and Wilcoxon for paired samples. Anova test were used for parametric analyses. The correlation rate was calculated using Spearman’s test. The Spearman rank correlation coefficient (rs) was determined. The multiple stepwise forward regression analyses were done. All results were expressed as mean ± standard deviation (mean±SD) or median with lower and upper quartiles. Statistical significance was set at p<0.05.
RESULTS
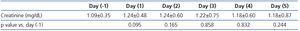
The blood count parameters after surgery were moderately, and yet significantly lower then before surgery (Table 1). These results indicate that blood loss and supplementation were not fully balanced during and after the operation, and, consequently, the lowered blood supply to the kidneys might have influenced kidney function, at least to a certain extent. After surgery, serum creatinine concentrations did not increase significantly. However, on the first day after surgery the “p” value of creatinine rise approximated 0.05 (Table 3). GFRs, estimated before surgery according to MDRD and CKD-EPI Study equations were 72.24±23.18 and 86.63±26.23mL/min/1.73m2, respectively.
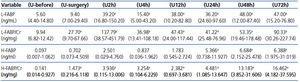
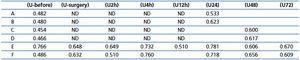
Urine L-FABP expressed as concentrations or urinary excretions normalized to urinary creatinine (L-FABP/Cr) increased significantly at almost all successive time points compared to day (-1). A significant increase in urine H-FABP concentrations was delayed until after 24 hours. However, when expressed as urinary excretions normalized to urinary creatinine (H-FABP/Cr), the significant increases in H-FABP were observed at all successive time points compared to day (-1) (Table 4).
Using the multiple stepwise forward regression methods we analysed the influence of independent values such as: age, smoking, diabetes, coronary artery disease (CAD), hypertension, peripheral vascular disease, taking of ACE-I or nonsteroid anti-inflammatory drugs (NAID), aneurysm diameter, time since last radiocontrast administration, operative time, aorta cross-clamping time, blood loss during surgery, Mehran score14 on the dependent values: L-FABP/Cr and H-FABP/Cr determined in the last time-point of observation (U72h). We found that: a. CAD (β=0.61; p<0.01), age (β=- 0.55; p<0.01), time since last radiocontrast administration (β=-0.39; p=0.04) and blood loss during surgery (β=-0.54; p=0.04) explained 52.38% of the variation in L-FABP/Cr as measured by the coefficient of determination R2 (F(7,12)=3.98; p=0.02), b. NLPZ (β=0.60; p<0.01), peripheral vascular disease (β=-0.57; p<0.01) and CAD (β=0.51; p=0.02) explained 41.05% of the variation in H-FABP/Cr as measured by the coefficient of determination R2 (F(5,15)=3.79; p=0,02).
Serum creatinine concentrations were correlated with urine L-FABP and H-FABP concentrations and also with L-FABP/Cr and H-FABP/Cr determined at the same successive time-points. Significant correlations were found on day (-1) and, randomly, 24 and 48 hours after surgery (Table 5).
L-FABP concentrations and L-FABP/Cr excretions were also correlated with H-FABP concentrations and H-FABP/Cr excretions at the same time-points, respectively. Except for correlation between L-FABP/Cr and H-FABP/Cr determined 12 hours after clamp release, all the other “rs” values were significant as presented in the Table 5.
No significant correlations were found between the time of aorta clamping (aorta clamping should be regarded as the major event of AAA surgery that may lead to kidney injury) and serum creatinine, urine L-FABP and H-FABP concentrations, L-FABP/Cr and H-FABP/Cr at any time-point.
DISCUSSION
FABPs play a crucial role in the metabolism of long chain and very long chain free fatty acids (FFAs). In the kidneys, the albumin-bound FFAs are filtered through glomeruli and subsequently reabsorbed by proximal tubules. During massive proteinuria, FFAs, filtered in excess, overload the tubular epithelium. Furthermore, other damaging factors such as diabetes, ischemia, toxins or overexertion could result in accumulation of FFAs and their oxidation products in the cytoplasm of tubular cells.11,15 After peroxidation, FFAs induce an inflammatory response resulting in tubulointerstitial injury. FABP is an intracellular FFAs-carrier facilitating their physiological β-oxidation in the mitochondria or peroxisomes.16 Therefore vigorous FFAs metabolism stimulated by FABPs prevents their undesirable peroxidation. L-FABP also has affinity to FFA oxidation products thus acting as intracellular antioxidant.17 L-FABP is a regulator of genes controlling lipid metabolism.18
Urinary L-FABP (u-L-FABP) is a reliable indicator of AKI (the area under the receiver operating characteristic curve could reach 0.93).19 It has been used successfully for surveillance of AKI after cardiac surgery,20 in contrast medium-induced nephropathy,21,22 acute ischemic injury23 or in AKI of varying etiology.19 H-FABP also is a reliable indicator of tissue damage. It is an early and sensitive marker of heart injury in acute coronary syndromes, exceeding in this respect, myoglobin, creatine kinase-MB fraction (CK-MB) or troponine I.24,25 H-FABP has been confirmed as a sensitive indicator of toxic distal tubular injury, particularly after gentamicin administration.11 However, up till now and compared to L-FABP, H-FABP has been used very rarely for the assessment of kidney injury.
These two FABPs are biochemically and functionally very similar, yet specifically expressed in different segments of the nephron,11,26 thus providing a unique possibility to simultaneously investigate damage to proximal and distal tubules using akin “biochemical tools”. Hofstra has already used L-FABP and H-FABP for this purpose in patients with idiopathic membranous nephropathy.12
Our study revealed very early and impressive FABPs increases (already during surgery - just before aortic clamp removal: time-point [U-surgery]; Table 4), which persisted with different intensity throughout the study period.
Portilla et al. studied AKI in the population of children after cardiac surgery. In this study u-L-FABP already increased 4 hours after cardiopulmonary bypass and remained elevated until 12 hours afterwards. In the subgroup with AKI defined as a 50% increase in the serum creatinine, u-L-FABP rose about 95 times after 4 hours and about 45 times after 12 hours. In the non-AKI subgroup, the increases were 10-fold and about 6-fold respectively.20 Different results were obtained in a study of contrast medium-induced nephropathy.21 u-L-FABP levels were determined in adults before and 1, 2 and 14 days after coronary angiography and showed a 1.7-2.5 -fold increase in the contrast-induced nephropathy subgroup (criterion of nephropathy: relative creatinine increase of 25% or more from baseline) during the observation period, but remained stable in the non-nephropathy subgroup. The results suggest that there is a strong discrepancy concerning the intensity of u-L-FABP excretion between these two studies. After cardiac surgery, u-L-FABP was considerably elevated even in the non-AKI group. In contrast-induced nephropathy, despite creatinine elevation, u-L-FABP excretion rose only moderately, and not in the creatinine-stable subgroup. Strangely enough, in the study on AKI of varying etiology there were no statistical differences in u-L-FABP among diagnostic categories, including contrast nephropathy.19 In our study population the creatinine level did not increase significantly after surgery (however, on day 1 after surgery, the p value corresponding to moderate serum creatinine elevation reached 0.095). One of the possible reasons of the lack of creatinine rise, particularly on the first day after surgery, could be the blood dilution. The blood dilution after the surgery is testified by the significant fall of hematocrite (summary effect of blood loss and crystalloids transfusion) (Table 1). u-L-FABP factored for creatinine excretion already increased during AAA surgery (U-surgery, Table 4), grew abruptly at 2 hours after aortic clamp release, formed more or less a plateau between 4 and 48 hours, but continued to increase at 72 hours of observation. The plateau reaction was observed in the contrast medium-induced nephropathy, but was very stable until the 14th day after angiography.21 On the contrary, after cardiac surgery, the peak excretion was already observed at 4 hours of intervention, followed by a distinct decline. Thus, our results seem to confirm the observations made in contrast nephropathy study and prove long-term stable reaction of u-L-FABP after kidney injury. From the other side, the very early first peak value of FABPs observed in our study in the 2nd hour after the aorta clamp release (U2h) is consistent with the very early peak excretion noted after cardiac surgery.20 The reason for this bi-phasic reaction of u-FABPs in our study (early peak in 2nd hour after intervention, then plateau on lower level with steady tendency to increase, Table 4) is not easy to explain. It is possible that the early peak in 2nd hour is caused by the process of sudden secretion of constitutively stored supplies of FABPs in tubular cells. As for the further plateau and steady increase of excretion, it might originate from the constantly intensifying process of “de novo” synthesis of FABPs.
The intensity of u-L-FABP response in our population was rather moderate compared to the two studies mentioned above.
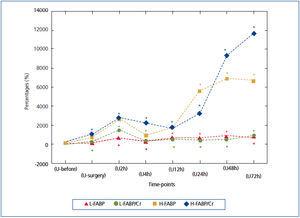
As remarked earlier, L-FABP and H-FABP are variants of the same substance performing, supposedly, similar physiological and pathophysiological role.9,11,16,27 Thus, both FABPs create a relatively homogenous tool for the investigation of two different segments of the nephron. These two FABPs were determined simultaneously in idiopathic membranous nephropathy.12 u-L-FABP correlated strongly with urinary H-FABP (u-H-FABP), r=0.88. We also found relatively strong correlations between FABPs, ranging from rs=0.486 to rs=0.781, at different time-points. This fact confirms that the putative damage to both segments of the nephron would occur simultaneously (Table 5). Despite the positive correlation between both FABPs at all time-points, their dynamics were rather different, as shown in Table 4. Nevertheless, each marker had a plateau in the middle phase of observation (between hour 4 and 48 for u-L-FABP and between hour 2 and 24 for u-H-FABP), and showed a strong tendency to increase in the final phase of observation. This might indicate a tendency to rise beyond the time of observation (Table 4). The magnitude of u-FABPs increases might correspond to the severity of tubular injury. Hence the need for a more detailed comparison of their dynamics. The direct juxtaposition is impossible, because the baseline values of L-FABP/Cr and H-FABP/Cr (determined before surgery: time-point-U-before) differ in factor 70.5 (9.94 vs. 0.141ng/mg) (Table 4). Therefore, we present also the increases as percentages of baseline values (Figure 1). The authors of the study on idiopathic membranous nephropathy suggest that the proximal tubule is injured earlier and stronger than the distal one, so an involvement of the distal tubule should be considered a sign of a more advanced pathology.12 This seems consistent with Eisei Noiri’s opinion that in the renal ischemia/reperfusion model the proximal tubules are much more sensitive to the hypoxic stress than the distal ones.28 Our analysis of percentage increases of both u-FABSs makes us draw an opposite conclusion, ie., that, following AAA surgery, the distal segment of the nephron could be more susceptible to injury than the proximal one.
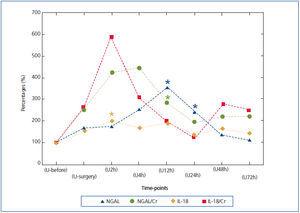
In order to compare u-FABP dynamics with other urinary AKI markers, we adapted the data from our previous publication29 in which the urinary excretions of neutrophil gelatinase-associated lipocalin (NGAL) and interleukin-18 (IL-18) were described. The NGAL and IL-18 were determined strictly according to the same protocol during AAA surgery, however in the smaller population comprising 14 subjects. As can be seen from Figure 2, in which analogically to the Figure 1 the percentage changes of NGAL and IL-18 baseline values (U-before) during successive time-points are shown, the dynamics was somewhat different. The rises of NGAL and IL-18 in comparison to both FABPs are less impressive and the absolute peak values appeared between 2nd and 12th hour after declamping of aorta. Strangely enough, the peak value of IL-18 excretion coincided exactly with the first peak value of FABPs observed in the 2nd hour after the aorta clamp release (U2h). The values of NGAL and IL-18 noted in further time-point were erratic and distinctly lower as compared to their early peak values. In contrast to them, the second u-H-FABP impressive rise in the later time-points (U24-U72) was observed. Therefore this juxtaposition of FABPs with NGAL and IL-18 determined in the same conditions suggest that FABPs reacts to tubular injury equally early but distinctly stronger (percentage increases) and more persistently.
We found positive, moderate correlations between FABPs and serum creatinine levels at baseline, which were not so apparent following AAA surgery (Table 5). These results suggest that under physiological conditions there is a link between GFR and u-FABPs levels. This link is partially lost after AAA surgery, maybe as an expression of ensuing kidney damage (disparate changes in tubular function and glomerular filtration).
As mentioned above, the serum creatinine level was non-significantly elevated in our study population. In historical perspective, the first notion describing sudden and mostly transitory kidney damage was “acute renal failure” and/or “acute tubular necrosis”. Later on a wider term “acute renal injury” was formed based on the RIFLE or AKIN criteria.30,31 This evolution was associated with the recognition of the fact that even small rises in serum creatinine level in different clinical settings could have unfavourable clinical and also economic consequences.3,4,32 But the usefulness of serum creatinine as a biomarker for diagnosis of AKI has been widely criticized. It has many disadvantages, as discussed elsewhere, and is therefore a suboptimal biomarker of kidney tissue injury. In fact, serum creatinine is a marker of kidney function loss (glomerular filtration), but not a direct indicator of kidney tissue injury.19,33 Moreover, chronic progressive renal failure correlates better with the intensity of tubulointerstitial damage than with glomerular injury.34,35 This situation, as a matter of fact, gave rise to a search for new, more sensitive biomarkers of AKI which led to the discovery of novel AKI indicators like L-FABP, H-FABP, Kidney Injury Molecule 1, NGAL, IL-18 or others. In our study, despite of no changes in serum creatinine levels, we found very early, strong and persistent rise of u-FABPs. Similar results were also found by other authors, ie., significant changes in kidney injury markers without changes in serum creatinine levels.22,36 We hypothesize that our results relate to the very early phase of AKI or, to use the RIFLE-AKIN criteria, the “pre-AKI” stage. Therefore a question arises concerning clinical sequelae of this “pre-AKI” condition for the general health status of these patients - a subject for further investigations. There is also an urgent need to redefine the term “acute kidney injury”. Currently, new direct indicators of renal tissue injury are available, performing without doubt much better than serum creatinine. But up till now, there is no “gold standard” of kidney injury. Histology result might be referred to;37 however this approach seems to be quite impractical from the clinical point of view. This important methodological problem should remain a subject of further discussion.
We conclude that very early, significant and persistent rise of both u-L-FABP and u-H-FABP after elective AAA surgery could indicate the damage of renal proximal and distal tubule in our study population. The obtained results also suggest that after AAA surgery the distal tubules could be more affected than the proximal ones. The u-FABPs could serve as very sensitive and early biomarkers of kidney injury and may allow to detect the very early and subtle phases of AKI (or “pre-AKI”), yet not reflected by increases of serum creatinine levels.
Conflict of interest
The authors declare they have no potential conflicts of interest related to the contents of this article. The study was funded by the Medical University of Silesia – grant No KNW-1-147/P/1/0.
Table 1. Patients characteristics
Table 2. Surgery details
Table 3. Serum creatinine levels on successive days
Table 4. Urine concentrations and urinary excretion normalized to urinary creatinine of L-FABP and H-FABP at successive time points
Table 5. Correlations
Figure 1. Urinary FABPs in successive time-points expressed as the percentage changes of baseline value: (U-before)=100%
Figure 2. Urinary NGAL and IL-18 in successive time-points expressed as the percentage changes of baseline value: (U-before)=100%