Introduction: A recent report by the CKD-EPI Chronic Kidney Disease Epidemiology Collaboration) group describes a new equation to estimate the glomerular filtration rate (GFR). This equation has been developed from a population of 8,254 subjects who had the GFR measured by iothalamate clearance (mean 68 ml/min/1.73 m2, SD 40 ml/min/1.73 m2). It includes variables such as serum creatinine, age, sex and race with different formula according to race, sex and creatinine value. The CKD-EPI equation improved the accuracy and precision results of the current first-choice MDRD-IDMS (Modification of Diet in Renal Disease-Isotopic Dilution Mass Spectrometry) formula, specially for GFR >60 ml/min/1.73 m2 in a group of 3,896 subjects. Methods: The goal of our study was to compare the estimated GFR by using the new equation CKD-EPI with MDRD-IDMS in a wide cohort of 14,427 patients (5,234 women and 9,193 men), and to analyze the impact of the new CKD-EPI formula on the staging of patients with CKD. Results: Mean estimated GFR was 0.6 ml/min/1.73 m2 higher with CKD-EPI as compared to MDRD-IDMS for the whole group, 1.9 ml/min/1.73 m2 higher for women and 0.2 ml/min/1.73 m2 lower for men. The percentage of CKD staging concordancy between equations varied from 79.4 % for stage 3A and 98.6% for stage 5. For those patients younger than 70 years, 18.9 % and 24 % MDRD-IDMS stages 3B and 3A were reclasified as CKD 3A and 2 by CKDEPI, respectively. For the same stages in the group younger than 70 years, the percentage of reclasified patients increased up to 34.4% and 33.4%, respectively. Conclusion: The new CKD-EPI equation to estimate the GFR reclasifies an important number of patients to higher CKD stages (higher GFR), specially younger women, clasified as CKD stage 3 by MDRD-IDMS.
Introducción: recientemente el grupo CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) ha publicado una nueva ecuación de estimación del filtrado glomerular (FG) desarrollada a partir de una población de 8.254 individuos a los que se midió el FG mediante aclaramiento de iotalamato (media 68 ml/min/1,73 m2, DE 40 ml/min/1,73 m2), y que incluye como variables la creatinina sérica, la edad, el sexo y la raza, con distintas versiones en función de la etnia, el sexo y el valor de la creatinina. La ecuación de CKD-EPI mejoró los resultados en cuanto a exactitud y precisión de la ecuación de elección actual MDRD-IDMS (Modification of Diet in Renal Disease-Isotopic Dilution Mass Spectrometry) en especial para valores de FG superior a 60 ml/min/1,73 m2 en un grupo de 3.896 individuos. Material y métodos: el objetivo de nuestro estudio fue comparar los valores de FG estimado utilizando la nueva ecuación de CKD-EPI frente a MDRD-IDMS en una amplia cohorte de 14.427 pacientes (5.234 mujeres y 9.193 hombres) y analizar las repercusiones que el uso de CKD-EPI tendría a la hora de clasificar a la población en distintos estadios de enfermedad renal crónica (ERC) en función de su FG. Resultados: la media del FG estimado fue 0,6 ml/min/1,73 m2 más alto por CKD-EPI que por MDRDIDMS en el grupo total, 1,9 ml/min/1,73 m2 más alto en el grupo de mujeres y 0,2 ml/min/1,73 m2 más bajo para los hombres. El porcentaje de concordancias en cuanto a asignación de estadio de ERC por ambas ecuaciones osciló entre el 79,4% para el estadio de ERC 3A y el 98,6% para el estadio de ERC 5. Para individuos de edad inferior a 70 años, un 18,9 y un 24% asignados por MDRD-IDMS a estadios de ERC 3B y ERC 3A fueron reclasificados como ERC 3A y ERC 2 por CKD-EPI, respectivamente. Para los mismos estadios en el grupo de mujeres de menos de 70 años, el porcentaje de casos reclasificados por CKD-EPI ascendió hasta el 34,4 y el 33,4%, respectivamente. Conclusiones: la nueva ecuación de estimación del FG CKD-EPI reclasifica hacia estadios de valor de FG superior un importante número de individuos, en especial mujeres de edad inferior a 70 años, catalogados como ERC 3 por MDRD-IDMS.
INTRODUCTION
Different epidemiological studies have shown that chronic kidney disease (CKD) is an important public health problem.1-6 Its presence has been linked with a high risk of end-stage chronic kidney disease, cardiovascular disease and death.7 Data from the EPIRCE study show that the prevalence of CKD, considered where there is a glomerular filtration rate (GFR) below 60ml/min/1.73m2 (stages 3-5 without dialysis), is 6.5% of the Spanish population over 18 years of age.8-10 The best index for measuring kidney function is GFR. Given that measuring inulin clearance directly or through isotopic methods is complicated, expensive, and cannot be used in daily practice, GFR estimates based on equations that use serum creatinine and other variables such as age, sex, ethnic group and body area have become popular.11-13 These equations improve the poor correlation that appears between creatinine and GFR. At present, most medical societies,14-22 including the Spanish Society of Nephrology (SEN) and the Spanish Society of Clinical Biochemistry and Molecular Pathology (SEQC), recommend using the equation from the MDRD study (Modification of Diet in Renal Disease) to estimate GFR; the recommendation appeared in a Consensus Document on glomerular filtration estimates which our group helped to prepare.14 It states that MDRD is to be used provided that serum creatinine is determined by either the classic method (MDRD-4) or the preferable method MDRDIDMS, depending on whether or not the analytical method used to determine creatinine is traceable to the reference method using isotopic dilution mass spectrometry (IDMS).15 However, factors such as the formula’s derivation population (patients with a certain degree of CKD) and difficulties with the lack of standardisation for the serum creatinine measurement (the resolution of which is at an advanced stage) pose a problem for its applicability. Showing the exact numerical value for GFR results above 60 or 90ml/min/1.73m2 is not recommended, depending on the clinical practice guide you consult.14-23 For the same reason, we advocate the need to search for new renal function markers or new equations for estimating GFR which would give better results than the MDRD. The CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) is a research group within the US National Institute of Diabetes and Digestive and Kidney Disease (NIDDK). It was formed in order to develop and validate GFR estimation equations based on data from different studies. This group recently published a new equation24 called CKD-EPI, which was developed based on a population of 8,254 subjects whose GFR was measured using iothalamate clearance (mean, 68ml/min/1.73m2, SD = 40ml/min/1.73m2), which takes into account variables such as serum creatinine, age, sex and ethnic group. This equation has different versions depending on ethnic group, sex and creatinine value (Table 1). According to the same study, comparing CKD-EPI to MDRD-IDMS shows that the former produces better results, especially for GFR values above 60ml/min/1.73m2. Comparison with direct GFR measurements shows it to be more accurate and precise, and therefore the authors concluded that CKD-EPI could replace MDRD-IDMS in daily clinical practice. The purpose of this study is to compare estimated GFR values obtained using the new CKD-EPI equation with those from MDRD-IMDS in a large patient cohort and analyse the new equation’s effect on classifying the population into different CKD stages according to GFR.
MATERIAL AND METHODS
Patients
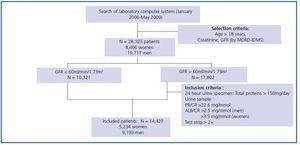
We used data from a cohort of 28,123 patients (8,406 women and 19,717 men) aged between 18 and 97 years whose creatinine serum was measured to evaluate renal function. Measurements took place in the Puigvert Foundation laboratory between January 2006 and May 2009. Puigvert Foundation is a centre of reference specialising in urology, nephrology and andrology. It is located at Santa Creu i Sant Pau University Hospital in Barcelona, and provides service to a population numbering approximately 450,000 inhabitants in the Barcelona metropolitan area. All of the creatinine results are accompanied by an estimated GFR calculated using the MDRD-IDMS method, in addition to a clinical commentary on the CKD stage, which is assigned according to the GFR value as per the recommendations in the Consensus Document on estimating glomerular filtration rate drawn up by the SEQC and the SEN.14 In the subject group with a GFR higher than 60ml/min/1.73m2 we only included cases presenting pathological proteinuria based on the total elimination of proteins in urine over 24 hours, the urine protein-tocreatinine or albumin-to-creatinine ratios in a morning sample, or the presence of proteins on the test strip in a random urine sample. The final cohort of patients included in the study contained 14,427 subjects: 5,234 women and 9,193 men. The patient selection and inclusion criteria are shown in Figure 1. Renal function was assessed on several occasions for some patients, which is why the total number of measurements included in the study reaches 51,579.
Method
Determining serum creatinine levels was done using a compensated kinetic Jaffe assay (Roche Diagnostics) which offers results that are traceable to the IDMS reference method. Values are expressed in μmol/l. Estimated GFR is calculated using the MDRD-IDMS and the CKD-EPI formulas, and values are expressed as ml/min/1.73m2. In urine, total proteins are measured by a turbidimetric assay with benzethonium chloride. Albumin is measured by immunoturbidimetric assay with polyclonal antibodies, and creatinine by a kinetic Jaffe method. All assays up to November 2007 were made using a Cobas Integra 700® chemistry analyser (Roche Diagnostics), and subsequent tests used a Cobas 6000® analyser (Roche Diagnostics). Assessing proteinuria from a test strip was carried out using the Combur Test® M system with an automatic reading given by a Miditron M® (Roche Diagnostics) urinalysis system; only those patients with proteinuria > 2+, corresponding to a concentration of 0.75g/l, were included. All of the biological quantities used in this study were subjected to internal and external quality control programmes, and all exceeded the analytical quality specifications recommended for their particular cases.
Statistical analysis
We calculated the mean and standard deviation for the values age, creatinine and GFR estimated by MDRD-IDMS (FGMDRD-IDMS) and by CKD-EPI (FGCKD-EPI) for the entire study population and for the population groups broken down by sex. The population was divided in CKD stages (1 to 5), using GFR obtained by the MDRD-IDMS as the reference value. Given the wide range and differing clinical meanings of stage 3 CKD, this stage was divided into substages 3A (GFR 45-59ml/min/1.73m2) and 3B (30-44ml/min/1.73m2) as recommended by some medical societies.16,19 We calculated the mean and the standard deviation of the GFR obtained for each stage with MDRD-IDMS and CKD-EPI. Using the Bland-Altman statistical process,25 we calculated the differences between the GFR values assigned for each of the formulas. These are expressed as absolute values (FGMDRD-IDMS – FGCKD-EPI, ml/min/1,73m2) and as percentage of differences ([(FGMDRD-IDMS – FGCKD-EPI )/ FGMDRD-IDMS] X 100, %). Lastly, we evaluated the percentage of concordance between the CKD stages assigned according to each of the formulas; where there was a discrepancy, we assessed how CKD-EPI reclassified subjects. All analyses were performed using SPSS Statistical Analysis® (version 17.0) and MedCalc® (MedCalc Software, version 8.1.0.0).
RESULTS
Table 2 shows the demographic characteristics of the study population and the values obtained for serum creatinine and GFR estimated using each of the formulas for the total subject group and the sex-specific groups. Glomerular filtration rate values were significantly different (p < 0.001) between men and women, whether by MDRD-IDMS or CKD-EPI. The mean estimated GFR was 0.6ml/min/1.73m2 higher with the CKD-EPI method in the total group, 1.9ml/min/1.73m2 higher for females, and 0.2ml/min/1.73m2 lower for males. The concordance results for the two formulas, referring to individuals who were classified in the same CKD stage by both methods with the stage assigned by the MDRD-IDMS method as the reference, ranged between 79.4% for stage 3A CKD and 98.6% for stage 5 CKD (Table 3) in the evaluation of the entire population. When the group was broken down by sex, we observed a higher concordance for the male group, with a range between 81.7% for stage 3A CKD and 99.2% for stage 5 CKD, and a lower one for the female group. Only 74.3% and 76.0% of the patients in CKD stages 3A and 2, respectively, were assigned to the same stage by both equations. Given the high number of elderly patients (42.2% were older than 70), we evaluated the weight of the age variable on the new equation, and observed a lower level of concordance between the two equations for patients younger than 70. The greatest differences between both equations could be observed when the sex and age variables were included simultaneously. Table 4 shows the number and percentage of cases with a concordant stage assignment for both equations (bold, in black), and the reclassification assigned by CKDEPI for non-concordant cases. For stage 4 and 5 CKD, the level of concordance was above 87% and 95% respectively, for all cases, regardless of age or sex. For stage 3B CKD, the greatest discrepancies were found in the group of women under 70; 24.7% of cases were reclassified as stage 3A CKD by CKD-EPI. For subjects in stage 3A, the new equation improved the CKD stage in the under-70 group regardless of sex. For stages with a GFR above 60ml/min/1.73m2 the performance of CKD-EPI compared with MDRD-IDMS was variable. We noted that more than 40% of the subjects older than 70 were moved from stage 1 CKD to stage 2 CKD. Table 5 shows the serum creatinine and GFR values assigned by both equations for different CKD stages, as well as the Bland-Altman analysis results. They are expressed as absolute values of the differences (ml/min/1.73m2) and as percentages, for the population total and for the sex-specific groups. A positive value indicates GFR values obtained from CKD-EPI are overestimated in comparison with those from MDRD-IDMS, and vice versa. Overall, the new equation obtained GFR values that were slightly lower for stage 4 to 5 CKD, and higher for the other stages. Analysis by sex showed that CKD-EPI generated higher GFR values in all stages (except for stage 5 CKD). We would like to point out an increase in GFR values of 8.5% and 9.2% for stages 2 and 3 CKD respectively in the female group.
DISCUSSION
The publication of the National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative (K/DOQI)16 guides in 2002 established the basis for defining and classifying CKD stages. According to K/DOQI criteria, CKD is understood as: The presence of GFR under 60ml/min/1.73m2 during a time period greater than or equal to three months. The presence of kidney damage, with or without a GFR decrease during a time period greater than or equal to three months, shown directly by histological abnormalities in the kidney biopsy, or indirectly by the presence of albuminuria, proteinuria, abnormal urinary sediment or an abnormal imagen studies. The combination of both diagnostic criteria is the base for CKD classification in 5 stages. Note that in initial stages (1 and 2), the GFR value itself is not a diagnostic marker; a marker associated with kidney damage must be present. Currently, different clinical practice guides for CKD recommend assessing GFR using equations based on creatinine measurements and different variables, such as age, sex or ethnic group. Although many equations have been published for this purpose, MDRD is the most widelyaccepted at this time. Use of the MDRD equation has led to major progress in the early diagnosis of CKD. This fact is accompanied by significant advantages, since early diagnosis allows us to start various treatments intended to halt or slow kidney disease progression and treat its complications (anaemia, secondary hyperparathyroidism, etc.) when they are in early stages. The ultimate goal is to improve care quality and patient survival.26-28 The MDRD equation, however, presents a set of limitations deriving from the population used to develop that measurement,12 which mostly consisted of individuals with differing degrees of CKD (mean GFR 40ml/min/1.73m2). Its lack of precision and systemic underestimating29-34 stand out, particularly for GFR values higher than 90ml/min/1.73m2. Underestimation may cause some individuals to be subjected to unnecessary examinations, receive underdoses when kidney-excreted drugs are prescribed, turned away from diagnostic imaging procedures that require the use of contrast and receive more aggressive treatments to lower cardiovascular risk factors. At the same time, the nearly non-existent representation of ethnic groups other than black or white in the population in which the formula was developed has given rise to the publication of equations with specific adjustment factors for other ethnic groups.35,36 For the same reason, we advocate the need for searching for new renal function markers or new equations for estimating GFR which would give better results than the MDRD, particularly for GFRs over 60ml/min/1.73m2. Cystatin C is an endogenous glomerular filtration marker that has been proposed as an alternative to creatinine and the formulas for estimating GFR to assess renal function. In recent years, numerous studies have been published comparing cystatin C’s potential as a GFR marker with that of creatinine. Most (but not all) of the studies state that cystatin C is a better marker. However, different formulas, developed in different populations, are available for estimating GFR based on cystatin. Comparing these equations with MDRD-4 or MDRD-IDMS produces heterogenous results.37-41 Today, regardless of the expectations that cystatin C may be a good GFR marker, particularly for high values, no clinical practice guide lists its use as a CKD parameter. Recently, the CKD-EPI published a new formula developed using a group of 8,254 participants in 10 clinical studies which included patients with differing clinical characteristics, with and without kidney disease and with a wide range of GFR values.24 All individuals included in the population from which the new formula was derived had their GFR measured by iothalamate clearance (mean 68ml/min/1.72m2, SD = 40ml/min/1.73m2), and serum creatinine values (mean 145μmol/l) were recalibrated according to the Roche enzymatic method that offers traceability to the IDMS reference method. The mean age of the population was 47 years, with a low representation of elderly patients; 9% of the subjects were aged 66 to 70 years, and only 3% were older than 71. The formula was obtained from a linear regression model for estimating the GFR logarithm based on the obtained creatinine levels and including the variables age, sex and ethnic group. Different versions of the formula exist for different ethnic groups, and different formulas exist within those groups depending on sex and creatinine level (Table 1). The CKD-EPI formula was subsequently validated in an independent population group with 3,896 individuals taken from 16 studies. Comparing the new formula with MDRD-IDMS makes it clear that CKD-EPI produces better results, especially for high GFR values. At the same time, it is as accurate as MDRD-IDMS for GFR values below 60ml/min/1.73m2 with a smaller deviation (median difference between measured and estimated GFRs of 2.5ml/min/1.73m2 vs 5.5ml/min/1.73m2), improved precision (interquartile range between differences = 16.6ml/min/1.73m2 vs 18.3 ml/min/1.73m2) and greater accuracy (percentage of estimated GFRs within 30% or less of measured GFR = 84.1% vs 80.6%). The use of CKD-EPI in the NHANES (1999-2006) showed that the median estimated GFR was 94.5ml/min/1.73m2 compared with 85.0 estimated using MDRD-IDMS, making CKD prevalence 11.5% rather than 13.1%; this drop in prevalence was basically produced by a decrease in the number of cases classified as stage 3 CKD by MDRD-IDMS. On the other hand, patient reclassification by CKD-EPI increased prevalence of stage 1 CKD while decreasing prevalence of stage 2 and 3 CKD. The spread of new formulas for evaluating GFR means that those formulas must be validated in populations with different clinical characteristics. The purpose of our study was to gather a significant number of patients with a wide range of GFR values in order to compare GFR results obtained using the MDRD-IDMS and the new CKD-EPI formula and analyse how this affected CKD stage classification. Our results indicate that the new formula delivers higher values than MDRD-IDMS does. This increase in GFR involves reclassifying patients in milder CKD stages; to this end, 9.8% of the cases that had been categorised as 3B CKD became 3A, 17% of 3A CKD cases became stage 2 CKD, and 15.7% went from stage 2 to stage 1 CKD. In addition, analysis by age subgroups showed that this tendency toward milder CKD stages was higher in the group younger than 70; 18.9% of the subjects went from stage 3B CKD to stage 3A, 34.1% from stage 3A to 2, and 24.0% from stage 2 to 1 CKD. Despite the fact that only 3.7% of the subjects included in the development of the CKD-EPI formula were older than 70, the percentage of concordance observed for this group in our study is greater than 90% for CKD stages 2 to 5; however, among those placed in CKD stage 1 by MDRDIDMS, a large number of cases were categorised as stage 2 CKD by the CKD-EPI formula. We believe that such a reassignment to a stage with a lower GFR is due to the MDRD-IDMS formula obtaining excessively high GFR values for some individuals in this population. These values are hard to believe given the age-related physiological decrease in GFR, and result from the low concentrations of serum creatinine that many of these patients present. The CKD-EPI formula is available in different versions depending on the creatinine level, as we see in Table 1. This is so that we can match results more closely to the true GFR value obtained by measuring iothalamate clearance. The most important aspect of this study is that it is the first publication attempting to validate the new CKD-EPI formula in our community, and that it was done in a large patient cohort. However, we must keep in mind that we do not know the true GFR value, since we do not have the means to measure it directly with a method of reference. However, this preliminary task was already carried out in the original publication, and so we believe that the results are largely comparable. Our results coincide with those obtained by Levey in that patients were reassigned to milder CKD stages, which is particularly true for the group categorised as stage 3 CKD by MDRD-IDMS. These results are due to characteristics of the CKD-EPI formula derivation population (individuals with mean GFR levels of 68ml/min/1.73m2, compared with 40ml/min/1.73m2 in the group used to develop the MDRD formula) and also to the use of standardised methods compared with the method of reference for measuring serum creatinine. The clinical practice guides that were recently written up by the SEN in conjunction with the Spanish society of family and community medicine (semFYC)42 state that referral to a nephrologist is advised for patients younger than 70 with a GFR below 45ml/min/1.73m2. Our results indicate that a high number of patients who are currently considered to be candidates for referral would no longer be candidates. This would have significant social health care consequences, as it would help reduce the congestion in nephrology units. Meanwhile, we can continue working to improve the accuracy and precision of GFR measurement and estimation methods. Furthermore, we believe that another important factor to consider when evaluating new estimated GFR formulas is assessment of their potential as prognostic factors for cardiovascular disease and/or survival.
Acknowledgements
This study was done with support from the Spanish Network of Neprhology Research (REDinREN) 16/06. RETICS. Carlos III Health Research Institute. Madrid, Spain.
Table 1. Estimated glomerular filtration rate formula CKD-EPI
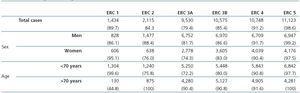
Table 2. Demographic characteristics of the population broken down by sex and age, with distribution by age group
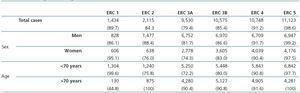
Table 3. Concordance in the CKD stage classifications for estimated glomerular filtration rates (GFR) using the MDRDIDMS and CKD-EPI formulas
Table 4. Concordances (shown in boldface black) in the classification of stages of chronic kidney disease (CKD) for glomerular filtration rate (GFR) estimated using the MDRD-IDMS and CKD-EPI formulas, taking GFR by MDRD-IDMS as a reference and considering sex and
Table 5. Values for serum creatinine and glomerular filtration rate estimated with the MDRD-IDMS (GFRMDRD-IDMS) and CKDEPI (GFRCKD-EPI) formulas for different stages of chronic kidney disease (CKD) and concordance analysis of GFRCKD-EPI and GFRMDRD-IDMS using the
Figure 1.