Simple renal cysts are uncommon lesions in paediatric patients. In the absence of hypokalaemia or an increase in the production of NH+, the cause of simple renal cysts is unknown. Hepler, in 1930, suggested that they may be caused by a tubular obstruction. We prospectively studied the presence of hypercalciuria or hypocitraturia as well as the family history of urolithiasis in a group of children diagnosed sonographically with simple renal cysts. The average age of the 22 patients (12M, 10F) was 6.04 ± 2.9 years at the time of diagnosis. The ultrasound examination had been requested due to urinary tract infection, abdominal pain, haematuria or other disorders. The cysts were slightly more frequent on the left side (54.5%). All were located in the upper kidney pole. 14 patients were found to have hypercalciuria and/or hypocitraturia (hypercalciuria n = 11, 50%). Thirteen families had history of renal stones. The metabolic abnormalities associated with calculi in children and/or family history of stones were present in 19 families (86.3%). Our hypothesis is that both entities, renal cysts, and genetic predisposition to kidney stones, are related.
Los quistes renales simples son lesiones poco frecuentes en pacientes pediátricos. En ausencia de hipopotasemia o de un incremento en la producción de NH4 +, se desconoce el origen de los mismos. Hepler, en 1930, propuso que su causa podría ser una obstrucción tubular. Hemos estudiado de forma prospectiva la presencia de hipercalciuria o de hipocitraturia, así como los antecedentes familiares de litiasis en un grupo de niños diagnosticados ecográficamente de quistes renales simples. Al diagnóstico, la edad media de los 22 pacientes (12 varones y 10 mujeres) fue de 6,04 ± 2,9 años. El estudio ecográfico se había solicitado por infección de vías urinarias, dolor abdominal, hematuria u otros. Los quistes fueron ligeramente más frecuentes en el lado izquierdo (54,5%). Todos estaban ubicados en el polo renal superior. En 14 pacientes (63,6%) se demostró que eran portadores de hipercalciuria o hipocitraturia (hipercalciuria n = 11, 50%). En 13 familias existían antecedentes de litiasis renal. En conjunto, las anomalías metabólicas estudiadas causantes de cálculos en los niños o los antecedentes familiares de litiasis estaban presentes en 19 familias (86,3%). Nuestra hipótesis es que ambas entidades, quistes renales y predisposición genética a padecer cálculos renales, están relacionadas.
INTRODUCTION
Simple renal cysts are common in adults. They are located in the cortex, are usually unilateral, and have increased incidence after 40 years of age.1,2 They are more frequent in males, left kidneys, and upper renal poles.3 However, they are rare in infants and children, in whom they usually appear as solitary lesions. Some that are detected prenatally tend to disappear, even before birth.1 They occasionally appear in multiple generations, such that some evidence has accumulated suggesting that it might be a rare form of a disease with an autosomal dominant pattern of inheritance.4
It has been suggested that these cysts originate from diverticula of the distal collecting duct. Therefore, these diverticula would increase in number in the kidneys of the elderly, probably as a result of thinning of the tubular basement membrane, which would explain the relationship between aging and simple renal cysts.1,5
Simple renal cysts rarely cause symptoms, although they have been occasionally associated with abdominal tumours or with flank pain.6 In general, the association with symptoms has been considered a simple coincidence.5 They are usually incidental ultrasound findings during the workup for urologyc problems, hypertension, or haematuria.6 In 1930, Hepler suggested that the aetiology of simple renal cysts may be tubular obstruction, such that they would increase in size over time.7 In this series, we wanted to know if simple renal cysts could be associated with a genetic predisposition for renal stone formation (prelithiasis). For that reason, we studied a group of children diagnosed with that entity to see if they were carriers of the two most common metabolic causes of urolithiasis (hypercalciuria and hypocitraturia) and if there was a history of urolithiasis in their families.
PATIENTS AND METHODS
Patients
We studied 22 patients (12 males and 10 females) with a mean age at diagnosis of 5.67 ± 3.03 years (range: 1-13 years), followed in the outpatient Paediatric Nephrology clinic of the Nuestra Señora de Candelaria University Hospital for a period of 1.77 ± 3.68 years (range: 0-17 years) (table 1). The ultrasound examination had been requested for abdominal pain (n = 6, 26.1%), acute pyelonephritis (n = 5, 21.7%), cystitis (n = 2, 8.7%), recurrent urinary tract infections (n = 2, 8.7%), microscopic haematuria (n = 1, 4.3%), macroscopic haematuria (n = 1, 4.3%), nocturnal enuresis (n = 2, 8.7%), and hyperactive bladder (n = 1, 4.3%). One patient suffering from distal renal tubular acidosis (DRTA) began to have vomiting and had weight below the 3rd percentile. The ultrasound for the remaining patient, who was asymptomatic, was requested for having two siblings with idiopathic hypercalciuria. The children’s parents were interviewed about any family history of urolithiasis.
Methods
In accordance with the criteria set by the Spanish Paediatric Nephrology Association, hypercalciuria was diagnosed in children between 1 and 2 years of age when the calcium/ creatinine quotient (UCa/UCr) from two isolated consecutive non-fasting urine samples was greater than 0.47mg/mg.8
Based on the results of So et al., hypercalciuria in children between 2 and 4 years of age was diagnosed when UCa/UCr was greater than 0.28mg/mg.9 In children older than 4 years of age, the diagnosis of hypercalciuria was made when the UCa/UCr value under similar conditions was greater than 0.20mg/mg,10 a number that corresponds to the 95th percentile of a sample made up of 100 healthy children from a previous study conducted by our group.11 Based on Stapleton and Kroovand’s criteria, the diagnosis of hypocitraturia was made when the value of the citrate/creatinine quotient was less than 400mg/g in two consecutively collected urine samples.12 The glomerular filtration rate (GFR) was calculated according to the Schwartz formula.13
Renal concentrating ability was determined with desmopressin (DDAVP). Before emptying the bladder, 20μg of intranasal desmopressin or 0.2mg (200μg) of desmopressin in tablet form was administered. Then, three urine samples were collected at 90-minute intervals. Food intake was allowed during this period, although subjects were advised not to drink too much fluid. The maximum value of any of the three samples was taken as the test result.
Measurements of calcium, citrate, and creatinine were made with standard techniques in an autoanalyser (Roche/Hitachi Modular®). Osmolality was determined measuring freezing point depression in an Osmostat® osmometer (Menarini). Ultrasound examinations were performed with an Orion® ultrasound (Philips).
Statistical methods
Statistical analysis was performed using the SPSS software package (version 17.0; SPSS, Chicago, IL). Basic statistics were performed and the Mann-Whitney U test was used when the sample was distributed in two subgroups. Probability values less than 0.05 were considered significant.
RESULTS
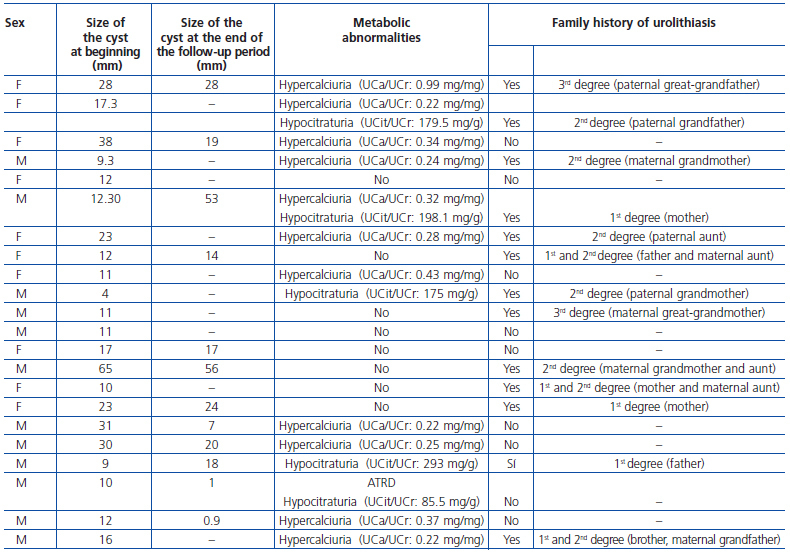
Simple renal cysts were most commonly located on the left side (n = 12, 54.5%), while 10 were seen on the right side (45.4%). They were all located in the upper renal pole. In the 12 patients who had two ultrasound scans available, the size of the cysts on diagnosis was 23.9 ± 16.1mm while it was 21.5 ± 17.5mm at the end of the follow-up period (NS) (table 1). Two patients had been diagnosed with vesicoureteral reflux. Nine patients were found to have hypercalciuria (40.9%), two had hypocitraturia (9.1%), two had hypercalciuria associated with hypocitraturia (9.1%), and one had hypocitraturia in the setting of a DRTA (4.5%). There was a family history of urolithiasis in 13 families (59.1%) (table 1). In eight of them, the children with renal cysts had hypercalciuria or hypocitraturia, and the other five did not. In short, if we combine the existence of hypercalciuria or hypocitraturia in the children and their family histories, we see that there is some manifestation of urolithiasis in some family member in 19 of the 22 families studied (86.3%).
Regarding renal function, only two patients (8.7%) had a defect in renal concentrating ability. None showed increased urinary elimination of microalbumin or decreased GFR. Furthermore, no patients had serum electrolyte abnormalities, except for the child with DRTA with hyperchloraemia on diagnosis (115mEq/l) (table 1).
When the sample was separated between patients with hypercalciuria or hypocitraturia and those with normal metabolic studies, differences were only found, obviously, in urinary calcium elimination. When the sample was separated between patients with a family history of urolithiasis and those without a family history, differences were seen in maximum urinary osmolality (951.6 ± 120.8 versus 1,059.1 ± 116.3mOsm/kg) (p = 0.04).
DISCUSSION
Simple renal cysts must meet three sonographic criteria in order to be specifically identified. The mass is round, with welldefined borders and smooth walls; there is an absence of interior echoes, and a strong echo is confirmed from the posterior wall, indicating good transmission through the cyst. On computerised axial tomography, the characteristics of a simple cyst are that it is neatly separated from the surrounding parenchyma, has a thin, smooth wall, the cystic fluid is homogeneous with density close to that of water, and there is no enhancement of the mass after administration of contrast.2 Simple renal cysts are rare in infancy. They are generally detected incidentally. In a large series reported by McHugh et al., in which 16,102 paediatric patients were studied by ultrasound, renal cysts were detected in 0.22%.14 In newborns and children up to 18 years of age, an incidence of 0.1 to 0.45% has been published.5 In contrast, the prevalence increases up to 11.9% in adults.6
In the natural history of simple renal cysts, it has been observed that their size can increase occasionally over time. In the previously mentioned study by McHugh et al., of 22 children who underwent ultrasound monitoring for 5 years, it was observed that the size had not changed in 17 of the cases (74%).14 In contrast, Terada et al., in a longitudinal study conducted in adults, reported a gradual progressive growth in the size and number of renal cysts, although more quickly in patients under 50 years old.6 In this series, we observed no difference in the size of the cysts during the follow-up period.
The precise mechanism of simple renal cyst formation is not well defined. Two possible mechanisms have been suggested. The first is an increase of intrarenal ammonia production. This induces cyst formation through various mechanisms, including complement activation, stimulation of synthesis of DNA and RNA, proteins, and various metabolic factors associated with renal ammoniogenesis.15 The second proposed mechanism is chronic hypokalaemia, which has been described as causing structural and functional anomalies, including increased growth of renal cells,16 a defect in renal concentrating ability, interstitial fibrosis or chronic inflammation, and formation of renal cysts.17 This type of cyst formation has been reported in primary hyperaldosteronism,18,19 DRTA,20-22 Bartter’s syndrome,23,24 and in apparent mineralocorticoid excess syndrome.25 Meanwhile, acquired renal cysts have been observed in chronic kidney disease.26 None of our patients had this last disease or hypokalaemia.
In our sample, 86.3% of patients (n = 19) had hypercalciuria, hypocitraturia, or a family history of kidney stones. The prevalence of hypercalciuria in our patients (n = 11, 50%) is much higher than what has been described in the general population. In a previously cited study, we reported that the prevalence of hypercalciuria in healthy children on the island of Tenerife was 3.8%.11 In other populations, the reported prevalence of hypercalciuria varies between 0.627 and 12.5%.28We are unaware of any description of the prevalence of hypocitraturia in a control infant population.
An interesting question related to the discussion of our results is the absence of hypercalciuria in some children with renal cysts despite a positive family history of urolithiasis. We must remember that daily urinary excretion of calcium depends on various factors, primarily diet, intestinal absorption of calcium, and bone formation rate. Any change in these factors can result in a change in calciuria. Furthermore, this parameter is modified not only by daily calcium intake but also by the intake of other nutrients, such as sodium, animal protein, whole grains, and omega-3 fatty acids. We have observed that some children suffering from familiar hypercalciuria show transient normalisation of calciuria around the age of puberty, probably due to increased body calcium requirements. In this sense, many of the children diagnosed with idiopathic hypercalciuria and osteopenia improved their bone mineral needs upon reaching puberty. 29 When puberty ends and growth has concluded, calcium excretion may increase once again. That phenomenon of transient decline in calciuria has also been observed in healthy adolescents.30 In short, if a child has a normal one-time metabolic study, the child may still have had at least hypercalciuria at a younger age.
However, the inverse may be true. That is to say, cases of hypercalciuria without a family history of urolithiasis may be due to temporary fluctuations in calcium elimination and not to true hypercalciuria.
Given that simple renal cysts are assumed to be acquired, a possible first causal mechanism could be mechanical, that is, that the cyst could occur secondary to an intratubular obstruction caused by crystalluria.
As previously mentioned, another aetiologic possibility for cyst formation is increased ammonia production.15 In patients with idiopathic hypercalciuria, progression to an incomplete distal tubular acidosis has been described.31 This is characterised, in the absence of spontaneous acidosis, by alkaline urine and urinary pH greater than 5.35, even after overload with CLNH4.31,32 However, urinary ammonia is not decreased but rather increased, which sets up, on the other hand, the absence of free hydrogen ions in the urine and makes decreasing the urinary pH impossible.30 In contrast, the elimination of titratable acidity is greatly reduced. Its origin lies in a proximal tubular dysfunction, in which ammonia synthesis would be stimulated, perhaps, by an intracellular acidosis of unknown origin. We have also shown the existence of this dysfunction of renal acidification capacity in children with idiopathic hypercalciuria.33
Our hypothesis is supported by the results of the study by Chang et al. in 2007.5 The frequency of urolithiasis in the group with cysts (n = 62) was 24.2% versus 11.5% in the control group (n = 515) (p < 0.001). Although these authors did not discuss any explanation in relation to their findings, they admitted that “renal stones may be a risk factor for the presence of simple renal cysts”.5
In short, whatever the cause, our hypothesis is that the two entities, renal cysts and a genetic predisposition to form renal calculi (prelithiasis), are related. Thus, the presence of simple renal cysts would join other well-known signs and symptoms that reveal the condition of carrying a genetic predisposition for kidney stone formation.34,35
New, larger studies, in particular in the adult population, are needed in order to demonstrate the association described in this study, as well as the possible pathological mechanisms involved.
Table 1. Ultrasound and metabolic characteristics of the study patients